Linking Metabolic Reprogramming, Plasticity and Tumor Progression
- PMID: 33673109
- PMCID: PMC7917602
- DOI: 10.3390/cancers13040762
Linking Metabolic Reprogramming, Plasticity and Tumor Progression
Abstract
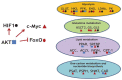
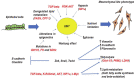
The specific molecular features of cancer cells that distinguish them from the normal ones are denoted as "hallmarks of cancer". One of the critical hallmarks of cancer is an altered metabolism which provides tumor cells with energy and structural resources necessary for rapid proliferation. The key feature of a cancer-reprogrammed metabolism is its plasticity, allowing cancer cells to better adapt to various conditions and to oppose different therapies. Furthermore, the alterations of metabolic pathways in malignant cells are heterogeneous and are defined by several factors including the tissue of origin, driving mutations, and microenvironment. In the present review, we discuss the key features of metabolic reprogramming and plasticity associated with different stages of tumor, from primary tumors to metastases. We also provide evidence of the successful usage of metabolic drugs in anticancer therapy. Finally, we highlight new promising targets for the development of new metabolic drugs.
Keywords: aerobic glycolysis; cancer metabolism; cancer therapy; lipid metabolism; metabolic reprograming; one-carbon metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Damaghi M., West J., Robertson-Tessi M., Xu L., Ferrall-Fairbanks M.C., Stewart P.A., Persi E., Fridley B.L., Altrock P.M., Gatenby R.A. The harsh microenvironment in early breast cancer selects for a Warburg phenotype. Proc. Natl. Acad. Sci. USA. 2020;118 doi: 10.1073/pnas.2011342118. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

