Cardiometabolism as an Interlocking Puzzle between the Healthy and Diseased Heart: New Frontiers in Therapeutic Applications
- PMID: 33673114
- PMCID: PMC7918460
- DOI: 10.3390/jcm10040721
Cardiometabolism as an Interlocking Puzzle between the Healthy and Diseased Heart: New Frontiers in Therapeutic Applications
Abstract
Cardiac metabolism represents a crucial and essential connecting bridge between the healthy and diseased heart. The cardiac muscle, which may be considered an omnivore organ with regard to the energy substrate utilization, under physiological conditions mainly draws energy by fatty acids oxidation. Within cardiomyocytes and their mitochondria, through well-concerted enzymatic reactions, substrates converge on the production of ATP, the basic chemical energy that cardiac muscle converts into mechanical energy, i.e., contraction. When a perturbation of homeostasis occurs, such as an ischemic event, the heart is forced to switch its fatty acid-based metabolism to the carbohydrate utilization as a protective mechanism that allows the maintenance of its key role within the whole organism. Consequently, the flexibility of the cardiac metabolic networks deeply influences the ability of the heart to respond, by adapting to pathophysiological changes. The aim of the present review is to summarize the main metabolic changes detectable in the heart under acute and chronic cardiac pathologies, analyzing possible therapeutic targets to be used. On this basis, cardiometabolism can be described as a crucial mechanism in keeping the physiological structure and function of the heart; furthermore, it can be considered a promising goal for future pharmacological agents able to appropriately modulate the rate-limiting steps of heart metabolic pathways.
Keywords: cardiac physiology; cardiometabolism; heart failure; ischemia; therapeutic targets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

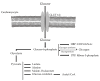
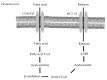
References
-
- Taegtmeyer H., Lam T., Davogustto G. Cardiac Metabolism in Perspective. Compr. Physiol. 2016;6:1675–1699. - PubMed
-
- Stanley W.C., Morgan E.E., Huang H., McElfresh T.A., Sterk J.P., Okere I.C., Chandler M.P., Cheng J., Dyck J.R., Lopaschuk G.D. Malonyl-CoA decarboxylase inhibition suppresses fatty acid oxidation and reduces lactate production during demand-induced ischemia. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H2304–H2309. doi: 10.1152/ajpheart.00599.2005. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

