Evaluation of Plaque Characteristics and Inflammation Using Magnetic Resonance Imaging
- PMID: 33673124
- PMCID: PMC7917750
- DOI: 10.3390/biomedicines9020185
Evaluation of Plaque Characteristics and Inflammation Using Magnetic Resonance Imaging
Abstract
Atherosclerosis is an inflammatory disease of large and medium-sized arteries, characterized by the growth of atherosclerotic lesions (plaques). These plaques often develop at inner curvatures of arteries, branchpoints, and bifurcations, where the endothelial wall shear stress is low and oscillatory. In conjunction with other processes such as lipid deposition, biomechanical factors lead to local vascular inflammation and plaque growth. There is also evidence that low and oscillatory shear stress contribute to arterial remodeling, entailing a loss in arterial elasticity and, therefore, an increased pulse-wave velocity. Although altered shear stress profiles, elasticity and inflammation are closely intertwined and critical for plaque growth, preclinical and clinical investigations for atherosclerosis mostly focus on the investigation of one of these parameters only due to the experimental limitations. However, cardiovascular magnetic resonance imaging (MRI) has been demonstrated to be a potent tool which can be used to provide insights into a large range of biological parameters in one experimental session. It enables the evaluation of the dynamic process of atherosclerotic lesion formation without the need for harmful radiation. Flow-sensitive MRI provides the assessment of hemodynamic parameters such as wall shear stress and pulse wave velocity which may replace invasive and radiation-based techniques for imaging of the vascular function and the characterization of early plaque development. In combination with inflammation imaging, the analyses and correlations of these parameters could not only significantly advance basic preclinical investigations of atherosclerotic lesion formation and progression, but also the diagnostic clinical evaluation for early identification of high-risk plaques, which are prone to rupture. In this review, we summarize the key applications of magnetic resonance imaging for the evaluation of plaque characteristics through flow sensitive and morphological measurements. The simultaneous measurements of functional and structural parameters will further preclinical research on atherosclerosis and has the potential to fundamentally improve the detection of inflammation and vulnerable plaques in patients.
Keywords: arterial elasticity; atherosclerosis; inflammation; magnetic resonance imaging; mouse models; pulse wave velocity; wall shear stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

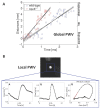
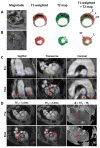
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

