Human Placenta Buffers the Fetus from Adverse Effects of Perceived Maternal Stress
- PMID: 33673157
- PMCID: PMC7918582
- DOI: 10.3390/cells10020379
Human Placenta Buffers the Fetus from Adverse Effects of Perceived Maternal Stress
Abstract
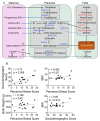
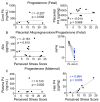
Maternal stress during pregnancy is linked to several negative birth outcomes. The placenta, a unique pregnancy-specific organ, not only nourishes and protects the fetus but is also the major source of progesterone and estrogens. As the placenta becomes the primary source of maternal progesterone (P4) and estradiol between 6-9 weeks of gestation, and these hormones are critical for maintaining pregnancy, maternal stress may modulate levels of these steroids to impact birth outcomes. The objective was to test whether maternal perceived stress crosses the placental barrier to modulate fetal steroids, including cortisol, which is a downstream indicator of maternal hypothalamic-pituitary-adrenal (HPA) axis regulation and is associated with negative fetal outcomes. Nulliparous women, 18 years or older, with no known history of adrenal or endocrine illness were recruited during their third trimester of pregnancy at the University of California San Francisco (UCSF) Mission Bay hospital obstetrics clinics. Simultaneous measurement of 10 steroid metabolites in maternal (plasma and hair) and fetal (cord blood and placenta) samples was performed using tandem mass spectrometry along with assessment of the perceived stress score and sociodemographic status. While the maternal perceived stress score (PSS) and sociodemographic status were positively associated with each other and each with the body mass index (BMI) (r = 0.73, p = 0.0008; r = 0.48, p = 0.05; r = 0.59, p = 0.014, respectively), PSS did not correlate with maternal or fetal cortisol, cortisone levels, or fetal birth weight. Regardless of maternal PSS or BMI, fetal steroid levels remained stable and unaffected. Progesterone was the only steroid analyte quantifiable in maternal hair and correlated positively with PSS (r = 0.964, p = 0.003), whereas cord estradiol was negatively associated with PSS (r = -0.94, p = 0.017). In conclusion, hair progesterone might serve as a better marker of maternal stress than cortisol or cortisone and maternal PSS negatively impacts fetal estradiol levels. Findings have implications for improved biomarkers of stress and targets for future research to identify factors that buffer the fetus from adverse effects of maternal stress.
Keywords: birth outcomes; cortisol; hair steroids; mass spectrometry; perceived stress.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Ding X.X., Wu Y.L., Xu S.J., Zhu R.P., Jia X.M., Zhang S.F., Huang K., Zhu P., Hao J.H., Tao F.B. Maternal anxiety during pregnancy and adverse birth outcomes: A systematic review and meta-analysis of prospective cohort studies. J. Affect. Disord. 2014;159:103–110. doi: 10.1016/j.jad.2014.02.027. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

