Fabry Disease: Molecular Basis, Pathophysiology, Diagnostics and Potential Therapeutic Directions
- PMID: 33673160
- PMCID: PMC7918333
- DOI: 10.3390/biom11020271
Fabry Disease: Molecular Basis, Pathophysiology, Diagnostics and Potential Therapeutic Directions
Abstract
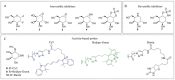
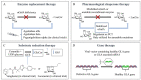
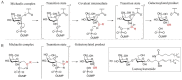
Fabry disease (FD) is a lysosomal storage disorder (LSD) characterized by the deficiency of α-galactosidase A (α-GalA) and the consequent accumulation of toxic metabolites such as globotriaosylceramide (Gb3) and globotriaosylsphingosine (lysoGb3). Early diagnosis and appropriate timely treatment of FD patients are crucial to prevent tissue damage and organ failure which no treatment can reverse. LSDs might profit from four main therapeutic strategies, but hitherto there is no cure. Among the therapeutic possibilities are intravenous administered enzyme replacement therapy (ERT), oral pharmacological chaperone therapy (PCT) or enzyme stabilizers, substrate reduction therapy (SRT) and the more recent gene/RNA therapy. Unfortunately, FD patients can only benefit from ERT and, since 2016, PCT, both always combined with supportive adjunctive and preventive therapies to clinically manage FD-related chronic renal, cardiac and neurological complications. Gene therapy for FD is currently studied and further strategies such as substrate reduction therapy (SRT) and novel PCTs are under investigation. In this review, we discuss the molecular basis of FD, the pathophysiology and diagnostic procedures, together with the current treatments and potential therapeutic avenues that FD patients could benefit from in the future.
Keywords: A4GALT; Fabry disease; enzyme replacement therapy; globotriaosyl-sphingosine (lysoGb3); globotriaosylceramide (Gb3); lysosomal storage disorders; pharmacological chaperone therapy; substrate reduction therapy; α-galactosidase A.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Anderson W. A Case of Angeo-Keratoma. Br. J. Dermatol. 1898;10:113–117. doi: 10.1111/j.1365-2133.1898.tb16317.x. - DOI
-
- Fabry J. Ein Beitrag zur Kenntniss der Purpura haemorrhagica nodularis (Purpura papulosa haemorrhagica Hebrae) Arch. Dermatol. Res. 1898;43:187–200. doi: 10.1007/BF01986897. - DOI
-
- Desnick R.J., Ioannou Y.A. In: α-Galactosidase a Deficiency. Fabry Disease. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. Scriver C.R., Beaudet A.L., Sly W.S., Valle D., editors. McGraw-Hill; New York, NY, USA: 2001.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

