Conventional and Unconventional Mechanisms by which Exocytosis Proteins Oversee β-cell Function and Protection
- PMID: 33673206
- PMCID: PMC7918544
- DOI: 10.3390/ijms22041833
Conventional and Unconventional Mechanisms by which Exocytosis Proteins Oversee β-cell Function and Protection
Abstract
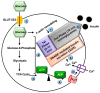
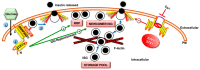
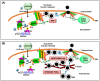
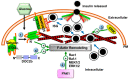
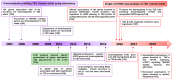
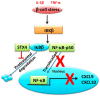
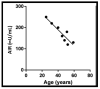
Type 2 diabetes (T2D) is one of the prominent causes of morbidity and mortality in the United States and beyond, reaching global pandemic proportions. One hallmark of T2D is dysfunctional glucose-stimulated insulin secretion from the pancreatic β-cell. Insulin is secreted via the recruitment of insulin secretory granules to the plasma membrane, where the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) and SNARE regulators work together to dock the secretory granules and release insulin into the circulation. SNARE proteins and their regulators include the Syntaxins, SNAPs, Sec1/Munc18, VAMPs, and double C2-domain proteins. Recent studies using genomics, proteomics, and biochemical approaches have linked deficiencies of exocytosis proteins with the onset and progression of T2D. Promising results are also emerging wherein restoration or enhancement of certain exocytosis proteins to β-cells improves whole-body glucose homeostasis, enhances β-cell function, and surprisingly, protection of β-cell mass. Intriguingly, overexpression and knockout studies have revealed novel functions of certain exocytosis proteins, like Syntaxin 4, suggesting that exocytosis proteins can impact a variety of pathways, including inflammatory signaling and aging. In this review, we present the conventional and unconventional functions of β-cell exocytosis proteins in normal physiology and T2D and describe how these insights might improve clinical care for T2D.
Keywords: DOC2b; STX4; exocytosis; insulin secretion; β-cell function; β-cell mass; β-cell senescence/aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

