A Toxic Synergy between Aluminium and Amyloid Beta in Yeast
- PMID: 33673244
- PMCID: PMC7918211
- DOI: 10.3390/ijms22041835
A Toxic Synergy between Aluminium and Amyloid Beta in Yeast
Abstract
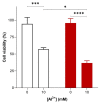
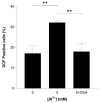
Alzheimer's disease (AD), the most prevalent, age-related, neurodegenerative disease, is associated with the accumulation of amyloid beta (Aβ) and oxidative stress. However, the sporadic nature of late-onset AD has suggested that other factors, such as aluminium may be involved. Aluminium (Al3+) is the most ubiquitous neurotoxic metal on earth, extensively bioavailable to humans. Despite this, the link between Al3+ and AD has been debated for decades and remains controversial. Using Saccharomyces cerevisiae as a model organism expressing Aβ42, this study aimed to examine the mechanisms of Al3+ toxicity and its interactions with Aβ42. S. cerevisiae cells producing Aβ42 treated with varying concentrations of Al3+ were examined for cell viability, growth inhibition, and production of reactive oxygen species (ROS). Al3+ caused a significant reduction in cell viability: cell death in yeast producing green fluorescent protein tagged with Aβ42 (GFP-Aβ42) was significantly higher than in cells producing green fluorescent protein (GFP) alone. Additionally, Al3+ greatly inhibited the fermentative growth of yeast producing GFP-Aβ42, which was enhanced by ferric iron (Fe3+), while there was negligible growth inhibition of GFP cells. Al3+- induced ROS levels in yeast expressing native Aβ42 were significantly higher than in empty vector controls. These findings demonstrate Al3+ has a direct, detrimental toxic synergy with Aβ42 that can be influenced by Fe3+, causing increased oxidative stress. Thus, Al3+ should be considered as an important factor, alongside the known characteristic hallmarks of AD, in the development and aetiology of the disease.
Keywords: Alzheimer’s disease; Fenton chemistry; aluminium; amyloid beta; iron; oxidative stress; yeast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

