Evidence for 2-Methoxyestradiol-Mediated Inhibition of Receptor Tyrosine Kinase RON in the Management of Prostate Cancer
- PMID: 33673346
- PMCID: PMC7918140
- DOI: 10.3390/ijms22041852
Evidence for 2-Methoxyestradiol-Mediated Inhibition of Receptor Tyrosine Kinase RON in the Management of Prostate Cancer
Abstract
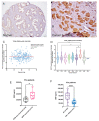
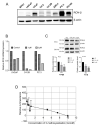
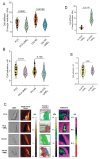
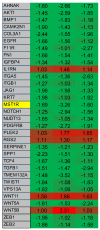
2-Methoxyestradiol (2-ME2) possesses anti-tumorigenic activities in multiple tumor models with acceptable tolerability profile in humans. Incomplete understanding of the mechanism has hindered its development as an anti-tumorigenic compound. We have identified for the first-time macrophage stimulatory protein 1 receptor (MST1R) as a potential target of 2-ME2 in prostate cancer cells. Human tissue validation studies show that MST1R (a.k.a RON) protein levels are significantly elevated in prostate cancer tissues compared to adjacent normal/benign glands. Serum levels of macrophage stimulatory protein (MSP), a ligand for RON, is not only associated with the risk of disease recurrence, but also significantly elevated in samples from African American patients. 2-ME2 treatment inhibited mechanical properties such as adhesion and elasticity that are associated with epithelial mesenchymal transition by downregulating mRNA expression and protein levels of MST1R in prostate cancer cell lines. Intervention with 2-ME2 significantly reduced tumor burden in mice. Notably, global metabolomic profiling studies identified significantly higher circulating levels of bile acids in castrated animals that were decreased with 2-ME2 intervention. In summary, findings presented in this manuscript identified MSP as a potential marker for predicting biochemical recurrence and suggest repurposing 2-ME2 to target RON signaling may be a potential therapeutic modality for prostate cancer.
Keywords: 2-methoxyestradiol; RON; atomic force microscopy; bile acids; castration-resistant prostate cancer; epithelial mesenchymal transition; mechanical properties; prostate cancer disparities; receptor tyrosine kinase.
Conflict of interest statement
Authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- American Cancer Society . Cancer Facts & Figures 2020. American Cancer Society; Atlanta, GA, USA: 2020. pp. 1–76.
-
- Du Y., Shen J., Hsu J.L., Han Z., Hsu M.-C., Yang C.-C., Kuo H.-P., Wang Y.-N., Yamaguchi H., Miller S.A., et al. Syntaxin 6-mediated Golgi translocation plays an important role in nuclear functions of EGFR through microtubule-dependent trafficking. Oncogene. 2014;33:756–770. doi: 10.1038/onc.2013.1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

