Molecular Imprinting Strategies for Tissue Engineering Applications: A Review
- PMID: 33673361
- PMCID: PMC7918123
- DOI: 10.3390/polym13040548
Molecular Imprinting Strategies for Tissue Engineering Applications: A Review
Abstract
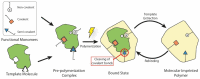
Tissue Engineering (TE) represents a promising solution to fabricate engineered constructs able to restore tissue damage after implantation. In the classic TE approach, biomaterials are used alongside growth factors to create a scaffolding structure that supports cells during the construct maturation. A current challenge in TE is the creation of engineered constructs able to mimic the complex microenvironment found in the natural tissue, so as to promote and guide cell migration, proliferation, and differentiation. In this context, the introduction inside the scaffold of molecularly imprinted polymers (MIPs)-synthetic receptors able to reversibly bind to biomolecules-holds great promise to enhance the scaffold-cell interaction. In this review, we analyze the main strategies that have been used for MIP design and fabrication with a particular focus on biomedical research. Furthermore, to highlight the potential of MIPs for scaffold-based TE, we present recent examples on how MIPs have been used in TE to introduce biophysical cues as well as for drug delivery and sequestering.
Keywords: molecularly imprinted polymers; scaffolds; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Molecularly Imprinted Intelligent Scaffolds for Tissue Engineering Applications.Tissue Eng Part B Rev. 2017 Feb;23(1):27-43. doi: 10.1089/ten.TEB.2016.0202. Epub 2016 Aug 31. Tissue Eng Part B Rev. 2017. PMID: 27484808
-
Progress in Molecularly Imprinted Polymers for Biomedical Applications.Comb Chem High Throughput Screen. 2019;22(2):78-88. doi: 10.2174/1386207322666190325115526. Comb Chem High Throughput Screen. 2019. PMID: 30914017 Review.
-
Molecularly imprinted beads by surface imprinting.Anal Bioanal Chem. 2007 Sep;389(2):369-76. doi: 10.1007/s00216-007-1362-4. Epub 2007 Jun 12. Anal Bioanal Chem. 2007. PMID: 17563884 Review.
-
Molecularly imprinted polymer for human viral pathogen detection.Mater Sci Eng C Mater Biol Appl. 2017 Aug 1;77:1341-1348. doi: 10.1016/j.msec.2017.03.209. Epub 2017 Mar 27. Mater Sci Eng C Mater Biol Appl. 2017. PMID: 28532011 Review.
-
Heterogeneity of Scaffold Biomaterials in Tissue Engineering.Materials (Basel). 2016 May 3;9(5):332. doi: 10.3390/ma9050332. Materials (Basel). 2016. PMID: 28773457 Free PMC article. Review.
Cited by
-
Recent molecularly imprinted polymers applications in bioanalysis.Chem Zvesti. 2023;77(2):619-655. doi: 10.1007/s11696-022-02488-3. Epub 2022 Sep 30. Chem Zvesti. 2023. PMID: 36213319 Free PMC article. Review.
-
Wearable biosensors for human fatigue diagnosis: A review.Bioeng Transl Med. 2022 May 17;8(1):e10318. doi: 10.1002/btm2.10318. eCollection 2023 Jan. Bioeng Transl Med. 2022. PMID: 36684114 Free PMC article. Review.
-
Regulation of cell fate by cell imprinting approach in vitro.Bioimpacts. 2024;14(3):29945. doi: 10.34172/bi.2023.29945. Epub 2023 Nov 28. Bioimpacts. 2024. PMID: 38938752 Free PMC article. Review.
-
A Fusion of Molecular Imprinting Technology and Siloxane Chemistry: A Way to Advanced Hybrid Nanomaterials.Nanomaterials (Basel). 2023 Jan 6;13(2):248. doi: 10.3390/nano13020248. Nanomaterials (Basel). 2023. PMID: 36677999 Free PMC article. Review.
-
Fabrication of Efficient and Non-Enzymatic Electrochemical Sensors for the Detection of Sucrose.Sensors (Basel). 2023 Feb 10;23(4):2008. doi: 10.3390/s23042008. Sensors (Basel). 2023. PMID: 36850609 Free PMC article.
References
-
- Hayden O., Lieberzeit P.A., Blaas D., Dickert F.L. Artificial antibodies for bioanalyte detection—Sensing viruses and proteins. Adv. Funct. Mater. 2006;16:1269–1278. doi: 10.1002/adfm.200500626. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

