Scrutinizing Mechanisms of the 'Obesity Paradox in Sepsis': Obesity Is Accompanied by Diminished Formation of Neutrophil Extracellular Traps (NETs) Due to Restricted Neutrophil-Platelet Interactions
- PMID: 33673387
- PMCID: PMC7918512
- DOI: 10.3390/cells10020384
Scrutinizing Mechanisms of the 'Obesity Paradox in Sepsis': Obesity Is Accompanied by Diminished Formation of Neutrophil Extracellular Traps (NETs) Due to Restricted Neutrophil-Platelet Interactions
Abstract
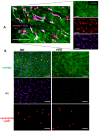
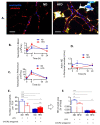
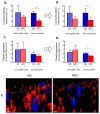
Systemic inflammation is a detrimental condition associated with high mortality. However, obese individuals seem to have higher chances of surviving sepsis. To elucidate what immunological differences exist between obese and lean individuals we studied the course of endotoxemia in mice fed high-fat diet (HFD) and ob/ob animals. Intravital microscopy revealed that neutrophil extracellular trap (NET) formation in liver vasculature is negligible in obese mice in sharp contrast to their lean counterparts (ND). Unlike in lean individuals, neutrophil influx is not driven by leptin or interleukin 33 (IL-33), nor occurs via a chemokine receptor CXCR2. In obese mice less platelets interact with neutrophils forming less aggregates. Platelets transfer from ND to HFD mice partially restores NET formation, and even further so upon P-selectin blockage on them. The study reveals that in obesity the overexaggerated inflammation and NET formation are limited during sepsis due to dysfunctional platelets suggesting their targeting as a therapeutic tool in systemic inflammation.
Keywords: endotoxemia; neutrophil extracellular traps; neutrophils; obesity; platelets; sepsis; systemic inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Metabolic Pathways Involved in Formation of Spontaneous and Lipopolysaccharide-Induced Neutrophil Extracellular Traps (NETs) Differ in Obesity and Systemic Inflammation.Int J Mol Sci. 2021 Jul 19;22(14):7718. doi: 10.3390/ijms22147718. Int J Mol Sci. 2021. PMID: 34299338 Free PMC article.
-
Reduced Neutrophil Extracellular Trap (NET) Formation During Systemic Inflammation in Mice With Menkes Disease and Wilson Disease: Copper Requirement for NET Release.Front Immunol. 2020 Jan 15;10:3021. doi: 10.3389/fimmu.2019.03021. eCollection 2019. Front Immunol. 2020. PMID: 32010131 Free PMC article.
-
Hydroxyethyl starch 130/0.4 decreases inflammation, neutrophil recruitment, and neutrophil extracellular trap formation.Br J Anaesth. 2015 Mar;114(3):509-19. doi: 10.1093/bja/aeu340. Epub 2014 Oct 16. Br J Anaesth. 2015. PMID: 25324349
-
The Evolving Role of Neutrophils and Neutrophil Extracellular Traps (NETs) in Obesity and Related Diseases: Recent Insights and Advances.Int J Mol Sci. 2024 Dec 20;25(24):13633. doi: 10.3390/ijms252413633. Int J Mol Sci. 2024. PMID: 39769394 Free PMC article. Review.
-
The Role of Platelet-Neutrophil Interactions in Driving Autoimmune Diseases.Immunology. 2025 May;175(1):1-15. doi: 10.1111/imm.13901. Epub 2025 Jan 18. Immunology. 2025. PMID: 39825744 Review.
Cited by
-
Itaconate Suppresses Formation of Neutrophil Extracellular Traps (NETs): Involvement of Hypoxia-Inducible Factor 1α (Hif-1α) and Heme Oxygenase (HO-1).Front Immunol. 2022 Jun 28;13:864638. doi: 10.3389/fimmu.2022.864638. eCollection 2022. Front Immunol. 2022. PMID: 35837403 Free PMC article.
-
PRE-ADMISSION BARIATRIC SURGERY IS ASSOCIATED WITH REDUCED MORTALITY IN SURGICAL PATIENTS WITH SEPSIS.Shock. 2025 Jun 1;63(6):844-850. doi: 10.1097/SHK.0000000000002568. Epub 2025 Mar 31. Shock. 2025. PMID: 40202402 Free PMC article.
-
Twelve-week high-fat diet for improving adverse ventricular remodeling post-myocardial infarction by alleviating local inflammation.Ann Transl Med. 2022 Oct;10(20):1089. doi: 10.21037/atm-22-1218. Ann Transl Med. 2022. PMID: 36388816 Free PMC article.
-
Dynamic regulation of neutrophil immunometabolism by platelet-derived metabolites.Front Immunol. 2025 Mar 27;16:1542438. doi: 10.3389/fimmu.2025.1542438. eCollection 2025. Front Immunol. 2025. PMID: 40213563 Free PMC article. Review.
-
Methods for the Assessment of NET Formation: From Neutrophil Biology to Translational Research.Int J Mol Sci. 2022 Dec 13;23(24):15823. doi: 10.3390/ijms232415823. Int J Mol Sci. 2022. PMID: 36555464 Free PMC article. Review.
References
-
- Singer M., Deutschman C.S., Seymour C., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) Jama J. Am. Med. Assoc. 2016;315:801–810. doi: 10.1001/jama.2016.0287. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

