MicroRNAs Modulate Signaling Pathways in Osteogenic Differentiation of Mesenchymal Stem Cells
- PMID: 33673409
- PMCID: PMC7956574
- DOI: 10.3390/ijms22052362
MicroRNAs Modulate Signaling Pathways in Osteogenic Differentiation of Mesenchymal Stem Cells
Abstract
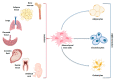
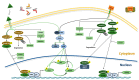
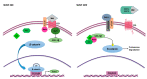
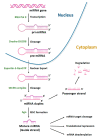
Mesenchymal stem cells (MSCs) have been identified in many adult tissues and they have been closely studied in recent years, especially in view of their potential use for treating diseases and damaged tissues and organs. MSCs are capable of self-replication and differentiation into osteoblasts and are considered an important source of cells in tissue engineering for bone regeneration. Several epigenetic factors are believed to play a role in the osteogenic differentiation of MSCs, including microRNAs (miRNAs). MiRNAs are small, single-stranded, non-coding RNAs of approximately 22 nucleotides that are able to regulate cell proliferation, differentiation and apoptosis by binding the 3' untranslated region (3'-UTR) of target mRNAs, which can be subsequently degraded or translationally silenced. MiRNAs control gene expression in osteogenic differentiation by regulating two crucial signaling cascades in osteogenesis: the transforming growth factor-beta (TGF-β)/bone morphogenic protein (BMP) and the Wingless/Int-1(Wnt)/β-catenin signaling pathways. This review provides an overview of the miRNAs involved in osteogenic differentiation and how these miRNAs could regulate the expression of target genes.
Keywords: MSCs; bone; bone regeneration; cell differentiation; mesenchymal stem cells; miRNA; miRNAome; microRNA; osteogenesis; osteogenic differentiation; stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31.Gene. 2013 Sep 15;527(1):321-31. doi: 10.1016/j.gene.2013.06.021. Epub 2013 Jul 1. Gene. 2013. PMID: 23827457
-
MicroRNA treatment modulates osteogenic differentiation potential of mesenchymal stem cells derived from human chorion and placenta.Sci Rep. 2021 Apr 7;11(1):7670. doi: 10.1038/s41598-021-87298-5. Sci Rep. 2021. PMID: 33828198 Free PMC article.
-
Post-Transcriptional Regulatory Crosstalk between MicroRNAs and Canonical TGF-β/BMP Signalling Cascades on Osteoblast Lineage: A Comprehensive Review.Int J Mol Sci. 2023 Mar 29;24(7):6423. doi: 10.3390/ijms24076423. Int J Mol Sci. 2023. PMID: 37047394 Free PMC article. Review.
-
A Novel OsteomiRs Expression Signature for Osteoblast Differentiation of Human Amniotic Membrane-Derived Mesenchymal Stem Cells.Biomed Res Int. 2019 Mar 24;2019:8987268. doi: 10.1155/2019/8987268. eCollection 2019. Biomed Res Int. 2019. PMID: 31019974 Free PMC article.
-
Roles for miRNAs in osteogenic differentiation of bone marrow mesenchymal stem cells.Stem Cell Res Ther. 2019 Jun 28;10(1):197. doi: 10.1186/s13287-019-1309-7. Stem Cell Res Ther. 2019. PMID: 31253175 Free PMC article. Review.
Cited by
-
Application of exosome-derived noncoding RNAs in bone regeneration: Opportunities and challenges.World J Stem Cells. 2022 Jul 26;14(7):473-489. doi: 10.4252/wjsc.v14.i7.473. World J Stem Cells. 2022. PMID: 36157529 Free PMC article. Review.
-
Bone Marrow Mesenchymal Stromal Cells in Multiple Myeloma: Their Role as Active Contributors to Myeloma Progression.Cancers (Basel). 2021 May 22;13(11):2542. doi: 10.3390/cancers13112542. Cancers (Basel). 2021. PMID: 34067236 Free PMC article. Review.
-
Regulatory mechanisms of circular RNAs during human mesenchymal stem cell osteogenic differentiation.Theranostics. 2024 Jan 1;14(1):143-158. doi: 10.7150/thno.89066. eCollection 2024. Theranostics. 2024. PMID: 38164139 Free PMC article. Review.
-
Mechanism of Hypoxia-Mediated Smooth Muscle Cell Proliferation Leading to Vascular Remodeling.Biomed Res Int. 2022 Dec 24;2022:3959845. doi: 10.1155/2022/3959845. eCollection 2022. Biomed Res Int. 2022. PMID: 36593773 Free PMC article. Review.
-
Accelerated reconstruction of rat calvaria bone defect using 3D-printed scaffolds coated with hydroxyapatite/bioglass.Sci Rep. 2023 Jul 27;13(1):12145. doi: 10.1038/s41598-023-38146-1. Sci Rep. 2023. PMID: 37500679 Free PMC article.
References
-
- Mazzoni E., D’Agostino A., Iaquinta M.R., Bononi I., Trevisiol L., Rotondo J.C., Patergnani S., Giorgi C., Gunson M.J., Arnett G.W., et al. Hydroxylapatite-Collagen Hybrid Scaffold Induces Human Adipose-Derived Mesenchymal Stem Cells to Osteogenic Differentiation in Vitro and Bone Regrowth in Patients. Stem Cells Transl. Med. 2020;9:377–388. doi: 10.1002/sctm.19-0170. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

