Can Serendipity Still Hold Any Surprises in the Coordination Chemistry of Mixed-Donor Macrocyclic Ligands? The Case Study of Pyridine-Containing 12-Membered Macrocycles and Platinum Group Metal Ions PdII, PtII, and RhIII
- PMID: 33673411
- PMCID: PMC7956204
- DOI: 10.3390/molecules26051286
Can Serendipity Still Hold Any Surprises in the Coordination Chemistry of Mixed-Donor Macrocyclic Ligands? The Case Study of Pyridine-Containing 12-Membered Macrocycles and Platinum Group Metal Ions PdII, PtII, and RhIII
Abstract
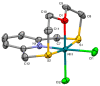
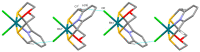
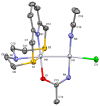
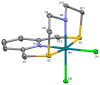
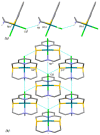
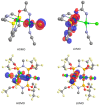
This study investigates the coordination chemistry of the tetradentate pyridine-containing 12-membered macrocycles L1-L3 towards Platinum Group metal ions PdII, PtII, and RhIII. The reactions between the chloride salts of these metal ions and the three ligands in MeCN/H2O or MeOH/H2O (1:1 v/v) are shown, and the isolated solid compounds are characterized, where possible, by mass spectroscopy and 1H- and 13C-NMR spectroscopic measurements. Structural characterization of the 1:1 metal-to-ligand complexes [Pd(L1)Cl]2[Pd2Cl6], [Pt(L1)Cl](BF4), [Rh(L1)Cl2](PF6), and [Rh(L3)Cl2](BF4)·MeCN shows the coordinated macrocyclic ligands adopting a folded conformation, and occupying four coordination sites of a distorted square-based pyramidal and octahedral coordination environment for the PdII/PtII, and RhIII complexes, respectively. The remaining coordination site(s) are occupied by chlorido ligands. The reaction of L3 with PtCl2 in MeCN/H2O gave by serendipity the complex [Pt(L3)(m-1,3-MeCONH)PtCl(MeCN)](BF4)2·H2O, in which two metal centers are bridged by an amidate ligand at a Pt1-Pt2 distance of 2.5798(3) Å and feature one square-planar and one octahedral coordination environment. Density Functional Theory (DFT) calculations, which utilize the broken symmetry approach (DFT-BS), indicate a singlet d8-d8 PtII-PtII ground-state nature for this compound, rather than the alleged d9-d7 PtI-PtIII mixed-valence character reported for related dinuclear Pt-complexes.
Keywords: DFT-Calculations; Rhodium; macrocyclic ligands; palladium; platinum.
Conflict of interest statement
The authors declare no conflict of interest and the funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures



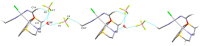

Similar articles
-
Spectroscopic and biological approach in the characterization of a novel 14-membered [N4] macrocyclic ligand and its palladium(II), platinum(II), ruthenium(III) and iridium(III) complexes.Spectrochim Acta A Mol Biomol Spectrosc. 2014 Jan 24;118:244-50. doi: 10.1016/j.saa.2013.08.079. Epub 2013 Sep 5. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24051297
-
Cyclometallated Pt(II) and Pd(II) complexes with a trithiacrown ligand.Dalton Trans. 2008 Apr 14;(14):1872-82. doi: 10.1039/b717910c. Epub 2008 Jan 15. Dalton Trans. 2008. PMID: 18369494
-
Structures, magnetochemistry, spectroscopy, theoretical study, and catechol oxidase activity of dinuclear and dimer-of-dinuclear mixed-valence Mn(III)Mn(II) complexes derived from a macrocyclic ligand.Inorg Chem. 2013 Jul 1;52(13):7732-46. doi: 10.1021/ic400916h. Epub 2013 Jun 10. Inorg Chem. 2013. PMID: 23750907
-
Pyridine alcohols: New applications reviving interest in metal complexes of a classical ligand.J Inorg Biochem. 2025 Aug;269:112880. doi: 10.1016/j.jinorgbio.2025.112880. Epub 2025 Mar 6. J Inorg Biochem. 2025. PMID: 40080992 Review.
-
Tuning metal-metal interactions for cooperative small molecule activation.Chem Commun (Camb). 2021 Mar 21;57(23):2839-2853. doi: 10.1039/d0cc07721f. Epub 2021 Feb 24. Chem Commun (Camb). 2021. PMID: 33624638 Free PMC article. Review.
Cited by
-
Coordination Chemistry of Mixed-Donor Pyridine-Containing Macrocyclic Ligands: From Optical to Redox Chemosensors for Heavy Metal Ions.Molecules. 2024 Dec 31;30(1):130. doi: 10.3390/molecules30010130. Molecules. 2024. PMID: 39795185 Free PMC article.
References
-
- Gloe K. Macrocyclic Chemistry, Current Trends and Future Perspectives. Springer; New York, NY, USA: 2005.
-
- Fitzpatrick D.W., Ulrich H.J. Macrocyclic Chemistry: New Research Developments (Chemistry Research and Applications) Nova Science Pub. Inc.; Hauppauge, NY, USA: 2010.
-
- Zolotov Y.A. Macrocyclic Compounds in Analytical Chemistry. Wiley & Sons; New York, NY, USA: 1997.
-
- Davis F., Higson S. Macrocycles: Construction, Chemistry and Nanotechnology Applications. Wiley; Chichester, UK: 2011.
-
- Marsault E., Peterson M.L. Practical Medicinal Chemistry with Macrocycles: Design, Synthesis, and Case Studies. Wiley; Chichester, UK: 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

