Circulating Tumor DNA Detection by Digital-Droplet PCR in Pancreatic Ductal Adenocarcinoma: A Systematic Review
- PMID: 33673558
- PMCID: PMC7956845
- DOI: 10.3390/cancers13050994
Circulating Tumor DNA Detection by Digital-Droplet PCR in Pancreatic Ductal Adenocarcinoma: A Systematic Review
Abstract
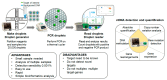
Pancreatic cancer (PC) is one of the most devastating malignant tumors, being the seventh leading cause of cancer-related death worldwide. Researchers and clinicians are endeavoring to develop strategies for the early detection of the disease and the improvement of treatment results. Adequate biopsy is still challenging because of the pancreas's poor anatomic location. Recently, circulating tumor DNA (ctDNA) could be identified as a liquid biopsy tool with huge potential as a non-invasive biomarker in early diagnosis, prognosis and management of PC. ctDNA is released from apoptotic and necrotic cancer cells, as well as from living tumor cells and even circulating tumor cells, and it can reveal genetic and epigenetic alterations with tumor-specific and individual mutation and methylation profiles. However, ctDNA sensibility remains a limitation and the accuracy of ctDNA as a biomarker for PC is relatively low and cannot be currently used as a screening or diagnostic tool. Increasing evidence suggests that ctDNA is an interesting biomarker for predictive or prognosis studies, evaluating minimal residual disease, longitudinal follow-up and treatment management. Promising results have been published and therefore the objective of our review is to understand the current role and the future perspectives of ctDNA in PC.
Keywords: ctDNA; digital-droplet PCR (ddPCR); pancreatic cancer; pancreatic ductal adenocarcinoma (PDAC).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Neoptolemos J.P., Palmer D.H., Ghaneh P., Psarelli E.E., Valle J.W., Halloran C.M., Faluyi O., O’Reilly D.A., Cunningham D., Wadsley J., et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

