Mitochondrial Transfer Improves Cardiomyocyte Bioenergetics and Viability in Male Rats Exposed to Pregestational Diabetes
- PMID: 33673574
- PMCID: PMC7956857
- DOI: 10.3390/ijms22052382
Mitochondrial Transfer Improves Cardiomyocyte Bioenergetics and Viability in Male Rats Exposed to Pregestational Diabetes
Abstract
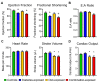
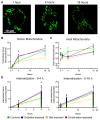
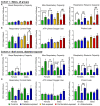
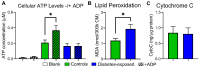
Offspring born to diabetic or obese mothers have a higher lifetime risk of heart disease. Previously, we found that rat offspring exposed to late-gestational diabetes mellitus (LGDM) and maternal high-fat (HF) diet develop mitochondrial dysfunction, impaired cardiomyocyte bioenergetics, and cardiac dysfunction at birth and again during aging. Here, we compared echocardiography, cardiomyocyte bioenergetics, oxidative damage, and mitochondria-mediated cell death among control, pregestational diabetes mellitus (PGDM)-exposed, HF-diet-exposed, and combination-exposed newborn offspring. We hypothesized that PGDM exposure, similar to LGDM, causes mitochondrial dysfunction to play a central, pathogenic role in neonatal cardiomyopathy. We found that PGDM-exposed offspring, similar to LGDM-exposed offspring, have cardiac dysfunction at birth, but their isolated cardiomyocytes have seemingly less bioenergetics impairment. This finding was due to confounding by impaired viability related to poorer ATP generation, more lipid peroxidation, and faster apoptosis under metabolic stress. To mechanistically isolate and test the role of mitochondria, we transferred mitochondria from normal rat myocardium to control and exposed neonatal rat cardiomyocytes. As expected, transfer provides a respiratory boost to cardiomyocytes from all groups. They also reduce apoptosis in PGDM-exposed males, but not in females. Findings highlight sex-specific differences in mitochondria-mediated mechanisms of developmentally programmed heart disease and underscore potential caveats of therapeutic mitochondrial transfer.
Keywords: developmentally programmed heart disease; diabetic pregnancy; mitochondria; mitochondrial transfer.
Conflict of interest statement
The authors declare no competing interest.
Figures
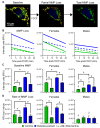
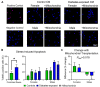
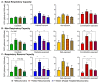
References
-
- Riskin A., Garcia-Prats J.A. Infants of women with diabetes. In: Weisman L.E., Wolfsdorf J.I., Kim M.S., editors. UpToDate. Wolters Kluwer; Online: 2020.
-
- Depla A.L., de Wit L., Steenhuis T.J., Slieker M.G., Voormolen D.N., Scheffer P.G., de Heus R., van Rijn B.B., Bekker M.N. Effects of maternal diabetes on fetal heart function at echocardiography: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2020 doi: 10.1002/uog.22163. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

