Targeting Toxins toward Tumors
- PMID: 33673582
- PMCID: PMC7956858
- DOI: 10.3390/molecules26051292
Targeting Toxins toward Tumors
Abstract
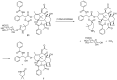
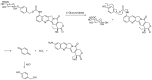
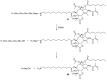
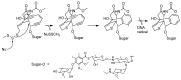
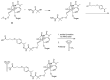
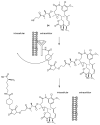
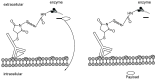
Many cancer diseases, e.g., prostate cancer and lung cancer, develop very slowly. Common chemotherapeutics like vincristine, vinblastine and taxol target cancer cells in their proliferating states. In slowly developing cancer diseases only a minor part of the malignant cells will be in a proliferative state, and consequently these drugs will exert a concomitant damage on rapidly proliferating benign tissue as well. A number of toxins possess an ability to kill cells in all states independently of whether they are benign or malignant. Such toxins can only be used as chemotherapeutics if they can be targeted selectively against the tumors. Examples of such toxins are mertansine, calicheamicins and thapsigargins, which all kill cells at low micromolar or nanomolar concentrations. Advanced prodrug concepts enabling targeting of these toxins to cancer tissue comprise antibody-directed enzyme prodrug therapy (ADEPT), gene-directed enzyme prodrug therapy (GDEPT), lectin-directed enzyme-activated prodrug therapy (LEAPT), and antibody-drug conjugated therapy (ADC), which will be discussed in the present review. The review also includes recent examples of protease-targeting chimera (PROTAC) for knockdown of receptors essential for development of tumors. In addition, targeting of toxins relying on tumor-overexpressed enzymes with unique substrate specificity will be mentioned.
Keywords: ADC; ADEPT; GDEPT; LEAPT; PROTAC; chemotherapy; drug targeting; overexpressed enzymes; prodrug.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
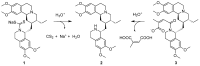
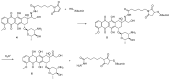





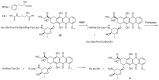

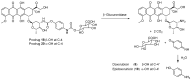




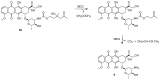

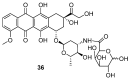
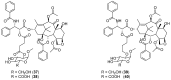
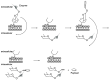
References
-
- Elsharif N.A. Review: Prodrug concept in drug design. Res. Rev. J. Pharm. Sci. 2018;9:22–28.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

