Prodigiosin Sensitizes Sensitive and Resistant Urothelial Carcinoma Cells to Cisplatin Treatment
- PMID: 33673611
- PMCID: PMC7957586
- DOI: 10.3390/molecules26051294
Prodigiosin Sensitizes Sensitive and Resistant Urothelial Carcinoma Cells to Cisplatin Treatment
Abstract
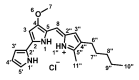
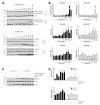
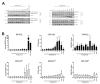
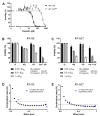
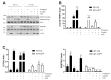
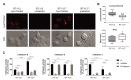
Cisplatin-based treatment is the standard of care therapy for urothelial carcinomas. However, complex cisplatin resistance mechanisms limit the success of this approach. Both apoptosis and autophagy have been shown to contribute to this resistance. Prodigiosin, a secondary metabolite from various bacteria, exerts different biological activities including the modulation of these two cellular stress response pathways. We analyzed the effect of prodigiosin on protein levels of different autophagy- and apoptosis-related proteins in cisplatin-sensitive and -resistant urothelial carcinoma cells (UCCs). Furthermore, we investigated the effect on cell viability of prodigiosin alone or in combination with cisplatin. We made use of four different pairs of cisplatin-sensitive and -resistant UCCs. We found that prodigiosin blocked autophagy in UCCs and re-sensitized cisplatin-resistant cells to apoptotic cell death. Furthermore, we found that prodigiosin is a potent anticancer agent with nanomolar IC50 values in all tested UCCs. In combination studies, we observed that prodigiosin sensitized both cisplatin-sensitive and -resistant urothelial carcinoma cell lines to cisplatin treatment with synergistic effects in most tested cell lines. These effects of prodigiosin are at least partially mediated by altering lysosomal function, since we detected reduced activities of cathepsin B and L. We propose that prodigiosin is a promising candidate for the therapy of cisplatin-resistant urothelial carcinomas, either as a single agent or in combinatory therapeutic approaches.
Keywords: apoptosis; autophagy; chemoresistance; cisplatin; prodigiosin; urothelial carcinoma.
Conflict of interest statement
The authors declare that there is no competing financial interest in relation to the work described.
Figures


Similar articles
-
Targeting urothelial carcinoma cells by combining cisplatin with a specific inhibitor of the autophagy-inducing class III PtdIns3K complex.Urol Oncol. 2018 Apr;36(4):160.e1-160.e13. doi: 10.1016/j.urolonc.2017.11.021. Epub 2017 Dec 21. Urol Oncol. 2018. PMID: 29276062
-
The marine triterpene glycoside frondoside A induces p53-independent apoptosis and inhibits autophagy in urothelial carcinoma cells.BMC Cancer. 2017 Feb 1;17(1):93. doi: 10.1186/s12885-017-3085-z. BMC Cancer. 2017. PMID: 28143426 Free PMC article.
-
Immunomodulatory proteins FIP-gts and chloroquine induce caspase-independent cell death via autophagy for resensitizing cisplatin-resistant urothelial cancer cells.Phytomedicine. 2016 Dec 1;23(13):1566-1573. doi: 10.1016/j.phymed.2016.09.003. Epub 2016 Sep 10. Phytomedicine. 2016. PMID: 27823620
-
Is Autophagy Always a Barrier to Cisplatin Therapy?Biomolecules. 2022 Mar 17;12(3):463. doi: 10.3390/biom12030463. Biomolecules. 2022. PMID: 35327655 Free PMC article. Review.
-
Relationships of Prodiginins Mechanisms and Molecular Structures to their Antiproliferative Effects.Anticancer Agents Med Chem. 2024;24(19):1383-1395. doi: 10.2174/0118715206314212240805105735. Anticancer Agents Med Chem. 2024. PMID: 39113301 Review.
Cited by
-
Excavatolide C/cisplatin combination induces antiproliferation and drives apoptosis and DNA damage in bladder cancer cells.Arch Toxicol. 2024 May;98(5):1543-1560. doi: 10.1007/s00204-024-03699-1. Epub 2024 Feb 29. Arch Toxicol. 2024. PMID: 38424264
-
NEURL1 acts as a candidate suppressor in bladder cancer by down-regulating PDE9A.In Vitro Cell Dev Biol Anim. 2025 Jun;61(6):681-693. doi: 10.1007/s11626-025-01047-w. Epub 2025 May 29. In Vitro Cell Dev Biol Anim. 2025. PMID: 40442542
-
Rise of the natural red pigment 'prodigiosin' as an immunomodulator in cancer.Cancer Cell Int. 2022 Dec 28;22(1):419. doi: 10.1186/s12935-022-02815-4. Cancer Cell Int. 2022. PMID: 36577970 Free PMC article. Review.
-
Recent Advances in Prodigiosin as a Bioactive Compound in Nanocomposite Applications.Molecules. 2022 Aug 5;27(15):4982. doi: 10.3390/molecules27154982. Molecules. 2022. PMID: 35956931 Free PMC article. Review.
-
Structures, biosynthesis, and bioactivities of prodiginine natural products.Appl Microbiol Biotechnol. 2022 Dec;106(23):7721-7735. doi: 10.1007/s00253-022-12245-x. Epub 2022 Nov 2. Appl Microbiol Biotechnol. 2022. PMID: 36319792 Review.
References
-
- WHO. [(accessed on 21 December 2020)]; Available online: https://gco.iarc.fr/today/online-analysis-table?v=2018&mode=cancer&mode_....
-
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Harnblasenkarzinoms, Langversion 2.0, 2020, AWMF-Registrierungsnummer 032/038OL (In German) [(accessed on 21 December 2020)]; Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/harnblasenkarzinom.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

