Selenium-Binding Protein 1 (SELENBP1) Supports Hydrogen Sulfide Biosynthesis and Adipogenesis
- PMID: 33673622
- PMCID: PMC7997437
- DOI: 10.3390/antiox10030361
Selenium-Binding Protein 1 (SELENBP1) Supports Hydrogen Sulfide Biosynthesis and Adipogenesis
Abstract
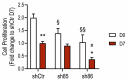
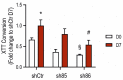
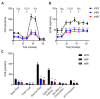
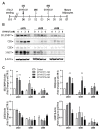
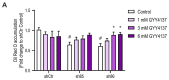
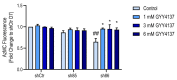
Hydrogen sulfide (H2S), a mammalian gasotransmitter, is involved in the regulation of a variety of fundamental processes including intracellular signaling, cellular bioenergetics, cell proliferation, and cell differentiation. Cystathionine g-lyase (CSE), cystathionine b-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST) are currently considered the three principal mammalian H2S-generating enzymes. However, recently, a fourth H2S-producing enzyme, selenium-binding-protein 1 (SELENBP1), has also been identified. The cellular regulatory role(s) of SELENBP1 are incompletely understood. The current study investigated whether SELENBP1 plays a role in the regulation of adipocyte differentiation in vitro. 3T3-L1 preadipocytes with or without SELENBP1 knock-down were subjected to differentiation-inducing conditions, and H2S production, cellular lipid accumulation, cell proliferation, and mitochondrial activity were quantified. Adipocyte differentiation was associated with an upregulation of H2S biosynthesis. SELENBP1 silencing decreased cellular H2S levels, suppressed the expression of the three "classical" H2S-producing enzymes (CBS, CSE, and 3-MST) and significantly suppressed adipocyte differentiation. Treatment of SELENBP1 knock-down cells with the H2S donor GYY4137 partially restored lipid accumulation, increased cellular H2S levels, and exerted a bell-shaped effect on cellular bioenergetics (enhancement at 1 and 3 mM, and inhibition at 6 mM). We conclude that SELENBP1 in adipocytes (1) contributes to H2S biosynthesis and (2) acts as an endogenous stimulator of adipocyte differentiation.
Keywords: fat; gasotransmitters, mitochondria, differentiation, metabolism; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

