Carbon Nanomaterials Embedded in Conductive Polymers: A State of the Art
- PMID: 33673680
- PMCID: PMC7957790
- DOI: 10.3390/polym13050745
Carbon Nanomaterials Embedded in Conductive Polymers: A State of the Art
Abstract
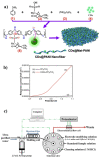
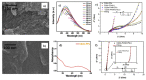
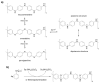
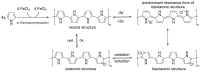
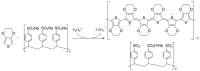
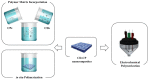
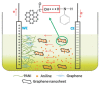
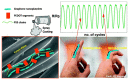
Carbon nanomaterials are at the forefront of the newest technologies of the third millennium, and together with conductive polymers, represent a vast area of indispensable knowledge for developing the devices of tomorrow. This review focusses on the most recent advances in the field of conductive nanotechnology, which combines the properties of carbon nanomaterials with conjugated polymers. Hybrid materials resulting from the embedding of carbon nanotubes, carbon dots and graphene derivatives are taken into consideration and fully explored, with discussion of the most recent literature. An introduction into the three most widely used conductive polymers and a final section about the most recent biological results obtained using carbon nanotube hybrids will complete this overview of these innovative and beyond belief materials.
Keywords: carbon dots; carbon nanotubes; conjugated polymers; graphene; poly(3,4-ethylenedioxythiophene), polypyrrole; polyaniline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Letheby H. On the production of a blue substance by the electrolysis of sulphate of aniline. J. Chem. Soc. 1862;15:161–163. doi: 10.1039/JS8621500161. - DOI
-
- De Surville R., Jozefowicz M., Yu L.T., Pepichon J., Buvet R. Electrochemical chains using protolytic organic semiconductors. Electrochim. Acta. 1968;13:1451–1458. doi: 10.1016/0013-4686(68)80071-4. - DOI
-
- Diaz A.F., Logan J.A. Electroactive polyaniline films. J. Electroanal. Chem. Interfacial Electrochem. 1980;111:111–114. doi: 10.1016/S0022-0728(80)80081-7. - DOI
-
- MacDiarmid A.G., Epstein A.J. Polyanilines: A novel class of conducting polymers. Faraday Discuss. Chem. Soc. 1989;88:317. doi: 10.1039/dc9898800317. - DOI
-
- Macdiarmid A.G., Chiang J.C., Richter A.F., Epstein A.J. Polyaniline: A new concept in conducting polymers. Synth. Met. 1987;18:285–290. doi: 10.1016/0379-6779(87)90893-9. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

