Fucoxanthin Suppresses Osteoclastogenesis via Modulation of MAP Kinase and Nrf2 Signaling
- PMID: 33673704
- PMCID: PMC7997314
- DOI: 10.3390/md19030132
Fucoxanthin Suppresses Osteoclastogenesis via Modulation of MAP Kinase and Nrf2 Signaling
Abstract
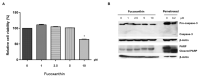
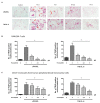
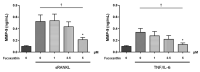
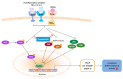
Fucoxanthin (FX), a natural carotenoid present in edible brown seaweed, is known for its therapeutic potential in various diseases, including bone disease. However, its underlying regulatory mechanisms in osteoclastogenesis remain unclear. In this study, we investigated the effect of FX on osteoclast differentiation and its regulatory signaling pathway. In vitro studies were performed using osteoclast-like RAW264.7 cells stimulated with the soluble receptor activator of nuclear factor-κB ligand or tumor necrosis factor-alpha/interleukin-6. FX treatment significantly inhibited osteoclast differentiation and bone resorption ability, and downregulated the expression of osteoclast-specific markers such as nuclear factor of activated T cells 1, dendritic cell-specific seven transmembrane protein, and matrix metallopeptidase 9. Intracellular signaling pathway analysis revealed that FX specifically decreased the activation of the extracellular signal-regulated kinase and p38 kinase, and increased the nuclear translocation of phosphonuclear factor erythroid 2-related factor 2 (Nrf2). Our results suggest that FX regulates the expression of mitogen-activated protein kinases and Nrf2. Therefore, FX is a potential therapeutic agent for osteoclast-related skeletal disorders including osteoporosis and rheumatoid arthritis.
Keywords: MAP kinase; Nrf2; brown seaweed; fucoxanthin; osteoclastogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

