Subversion of Niche-Signalling Pathways in Colorectal Cancer: What Makes and Breaks the Intestinal Stem Cell
- PMID: 33673710
- PMCID: PMC7957493
- DOI: 10.3390/cancers13051000
Subversion of Niche-Signalling Pathways in Colorectal Cancer: What Makes and Breaks the Intestinal Stem Cell
Abstract
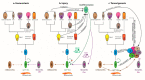
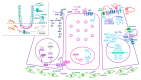
The intestinal epithelium fulfils pleiotropic functions in nutrient uptake, waste elimination, and immune surveillance while also forming a barrier against luminal toxins and gut-resident microbiota. Incessantly barraged by extraneous stresses, the intestine must continuously replenish its epithelial lining and regenerate the full gamut of specialized cell types that underpin its functions. Homeostatic remodelling is orchestrated by the intestinal stem cell (ISC) niche: a convergence of epithelial- and stromal-derived cues, which maintains ISCs in a multipotent state. Following demise of homeostatic ISCs post injury, plasticity is pervasive among multiple populations of reserve stem-like cells, lineage-committed progenitors, and/or fully differentiated cell types, all of which can contribute to regeneration and repair. Failure to restore the epithelial barrier risks seepage of toxic luminal contents, resulting in inflammation and likely predisposing to tumour formation. Here, we explore how homeostatic niche-signalling pathways are subverted in tumorigenesis, enabling ISCs to gain autonomy from niche restraints ("ISC emancipation") and transform into cancer stem cells capable of driving tumour initiation, progression, and therapy resistance. We further consider the implications of the pervasive plasticity of the intestinal epithelium for the trajectory of colorectal cancer, the emergence of distinct molecular subtypes, the propensity to metastasize, and the development of effective therapeutic strategies.
Keywords: BMP; Notch; Wnt; YAP; cancer stem cells (CSCs); colorectal cancer (CRC); consensus molecular subtypes (CMS); intestinal stem cell (ISC) niche; intestinal stem cells (ISCs); regeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Reserve Stem Cells in Intestinal Homeostasis and Injury.Gastroenterology. 2018 Nov;155(5):1348-1361. doi: 10.1053/j.gastro.2018.08.016. Epub 2018 Aug 15. Gastroenterology. 2018. PMID: 30118745 Free PMC article. Review.
-
Krüppel-like Factor 5 Regulates Stemness, Lineage Specification, and Regeneration of Intestinal Epithelial Stem Cells.Cell Mol Gastroenterol Hepatol. 2020;9(4):587-609. doi: 10.1016/j.jcmgh.2019.11.009. Epub 2019 Nov 25. Cell Mol Gastroenterol Hepatol. 2020. PMID: 31778829 Free PMC article.
-
Bile Acids Signal via TGR5 to Activate Intestinal Stem Cells and Epithelial Regeneration.Gastroenterology. 2020 Sep;159(3):956-968.e8. doi: 10.1053/j.gastro.2020.05.067. Epub 2020 May 30. Gastroenterology. 2020. PMID: 32485177
-
Gut microbiota-stem cell niche crosstalk: A new territory for maintaining intestinal homeostasis.Imeta. 2022 Sep 27;1(4):e54. doi: 10.1002/imt2.54. eCollection 2022 Dec. Imeta. 2022. PMID: 38867904 Free PMC article. Review.
-
Plasticity of Intestinal Epithelium: Stem Cell Niches and Regulatory Signals.Int J Mol Sci. 2020 Dec 31;22(1):357. doi: 10.3390/ijms22010357. Int J Mol Sci. 2020. PMID: 33396437 Free PMC article. Review.
Cited by
-
Implication of Obesity and Gut Microbiome Dysbiosis in the Etiology of Colorectal Cancer.Cancers (Basel). 2023 Mar 22;15(6):1913. doi: 10.3390/cancers15061913. Cancers (Basel). 2023. PMID: 36980799 Free PMC article. Review.
-
Epigenetic and Oncogenic Inhibitors Cooperatively Drive Differentiation and Kill KRAS-Mutant Colorectal Cancers.Cancer Discov. 2024 Dec 2;14(12):2430-2449. doi: 10.1158/2159-8290.CD-23-0866. Cancer Discov. 2024. PMID: 39121480 Free PMC article.
-
Inflammation-Associated Stem Cells in Gastrointestinal Cancers: Their Utility as Prognostic Biomarkers and Therapeutic Targets.Cancers (Basel). 2024 Sep 12;16(18):3134. doi: 10.3390/cancers16183134. Cancers (Basel). 2024. PMID: 39335106 Free PMC article. Review.
-
Targeting ligand-dependent wnt pathway dysregulation in gastrointestinal cancers through porcupine inhibition.Pharmacol Ther. 2022 Oct;238:108179. doi: 10.1016/j.pharmthera.2022.108179. Epub 2022 Mar 28. Pharmacol Ther. 2022. PMID: 35358569 Free PMC article. Review.
-
Understanding pre-metastatic niche formation: implications for colorectal cancer liver metastasis.J Transl Med. 2025 Mar 17;23(1):340. doi: 10.1186/s12967-025-06328-2. J Transl Med. 2025. PMID: 40098140 Free PMC article. Review.
References
-
- Sasaki N., Sachs N., Wiebrands K., Ellenbroek S.I., Fumagalli A., Lyubimova A., Begthel H., van den Born M., van Es J.H., Karthaus W.R., et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc. Natl. Acad. Sci. USA. 2016;113:E5399–E5407. doi: 10.1073/pnas.1607327113. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

