Tocilizumab improves survival in severe COVID-19 pneumonia with persistent hypoxia: a retrospective cohort study with follow-up from Mumbai, India
- PMID: 33673818
- PMCID: PMC7934984
- DOI: 10.1186/s12879-021-05912-3
Tocilizumab improves survival in severe COVID-19 pneumonia with persistent hypoxia: a retrospective cohort study with follow-up from Mumbai, India
Abstract
Background: Cytokine storm triggered by Severe Coronavirus Disease 2019 (COVID-19) is associated with high mortality. With high Interleukin -6 (IL-6) levels reported in COVID-19 related deaths in China, IL-6 is considered to be the key player in COVID-19 cytokine storm. Tocilizumab, a monoclonal antibody against IL-6 receptor, is used on compassionate grounds for treatment of COVID-19 cytokine storm. The aim of this study was to assess effect of tocilizumab on mortality due to COVID-19 cytokine storm.
Method: This retrospective, observational study included patients of severe COVID-19 pneumonia with persistent hypoxia (defined as saturation 94% or less on supplemental Oxygen of 15 L per minute through non-rebreathing mask or PaO2/FiO2 ratio of less than 200) who were admitted to a tertiary care center in Mumbai, India, between 31st March to 5th July 2020. In addition to standard care, single Inj. Tocilizumab 400 mg was given intravenously to 151 consecutive COVID-19 patients with persistent hypoxia, from 13th May to 5th July 2020. These 151 patients were retrospectively analysed and compared with historic controls, ie consecutive COVID-19 patients with persistent hypoxia, defined as stated above (N = 118, from our first COVID-19 admission on 31st March to 12th May 2020 i.e., till tocilizumab was available in hospital). Univariate and multivariate Cox regression analysis was performed for identifying predictors of survival. Statistical analysis was performed using IBM SPSS version 26.
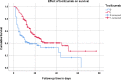
Results: Out of 269 (151 in tocilizumab group and 118 historic controls) patients studied from 31st March to 5th July 2020, median survival in the tocilizumab group was significantly longer than in the control group; 18 days (95% CI, 11.3 to 24.7) versus 9 days (95% CI, 5.7 to 12.3); log rank p 0.007. On multivariate Cox regression analysis, independent predictors of survival were use of tocilizumab (HR 0.621, 95% CI 0.427-0.903, P 0.013) and higher oxygen saturation.
Conclusion: Tocilizumab may improve survival in severe COVID-19 pneumonia with persistent hypoxia. Randomised controlled trials on use of tocilizumab as rescue therapy in patients of severe COVID-19 pneumonia with hypoxia (PaO2/FiO2 less than 200) due to hyperinflammatory state, are warranted.
Keywords: CO-RADS; CT-severity score; Cytokine storm; Hyperinflammatory syndrome; IL-6; Interlukin-6.
Conflict of interest statement
Authors have ‘No competing interests’ to declare.
Figures
Similar articles
-
Clinical characteristics and predictors of survival in adults with coronavirus disease 2019 receiving tocilizumab.J Autoimmun. 2020 Nov;114:102512. doi: 10.1016/j.jaut.2020.102512. Epub 2020 Jul 3. J Autoimmun. 2020. PMID: 32646770 Free PMC article.
-
Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial.Lancet Respir Med. 2021 May;9(5):511-521. doi: 10.1016/S2213-2600(21)00081-3. Epub 2021 Mar 4. Lancet Respir Med. 2021. PMID: 33676589 Free PMC article. Clinical Trial.
-
Treatment of severely ill COVID-19 patients with anti-interleukin drugs (COV-AID): A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jun 3;21(1):468. doi: 10.1186/s13063-020-04453-5. Trials. 2020. PMID: 32493441 Free PMC article.
-
Clinical outcomes in COVID-19 patients treated with tocilizumab: An individual patient data systematic review.J Med Virol. 2020 Nov;92(11):2516-2522. doi: 10.1002/jmv.26038. Epub 2020 Jun 9. J Med Virol. 2020. PMID: 32436994 Free PMC article.
-
Tocilizumab for severe COVID-19: a systematic review and meta-analysis.Int J Antimicrob Agents. 2020 Sep;56(3):106103. doi: 10.1016/j.ijantimicag.2020.106103. Epub 2020 Jul 23. Int J Antimicrob Agents. 2020. PMID: 32712333 Free PMC article.
Cited by
-
Effectiveness of Tocilizumab in COVID-19 Patients with Pneumonia: A Systematic Review.Acta Med Philipp. 2025 Jan 31;59(2):72-80. doi: 10.47895/amp.vi0.8188. eCollection 2025. Acta Med Philipp. 2025. PMID: 39967711 Free PMC article.
-
Beneficial and harmful outcomes of tocilizumab in severe COVID-19: A systematic review and meta-analysis.Pharmacotherapy. 2021 Nov;41(11):884-906. doi: 10.1002/phar.2627. Epub 2021 Oct 1. Pharmacotherapy. 2021. PMID: 34558742 Free PMC article.
-
Tocilizumab administration for the treatment of hospitalized patients with COVID-19: A systematic review and meta-analysis.Respirology. 2021 Nov;26(11):1027-1040. doi: 10.1111/resp.14152. Epub 2021 Oct 3. Respirology. 2021. PMID: 34605114 Free PMC article.
-
Single versus multiple doses of Tocilizumab in critically ill patients with coronavirus disease 2019 (COVID-19): A two-center, retrospective cohort study.J Crit Care. 2021 Dec;66:44-51. doi: 10.1016/j.jcrc.2021.08.007. Epub 2021 Aug 23. J Crit Care. 2021. PMID: 34438133 Free PMC article.
-
Cytokine storm and translating IL-6 biology into effective treatments for COVID-19.Front Med. 2023 Dec;17(6):1080-1095. doi: 10.1007/s11684-023-1044-4. Epub 2023 Dec 29. Front Med. 2023. PMID: 38157195 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical