Direct Structural Insights into GABAA Receptor Pharmacology
- PMID: 33674151
- PMCID: PMC8122054
- DOI: 10.1016/j.tibs.2021.01.011
Direct Structural Insights into GABAA Receptor Pharmacology
Abstract
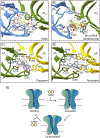
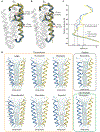
GABAA receptors are pentameric ligand-gated ion channels that mediate most fast neuronal inhibition in the brain. In addition to their important physiological roles, they are noteworthy in their rich pharmacology; prominent drugs used for anxiety, insomnia, and general anesthesia act through positive modulation of GABAA receptors. Direct structural information for how these drugs work was absent until recently. Efforts in structural biology over the past few years have revealed how important drug classes and natural products interact with the GABAA receptor, providing a foundation for studies in dynamics and structure-guided drug design. Here, we review recent developments in GABAA receptor structural pharmacology, focusing on subunit assemblies of the receptor found at synapses.
Keywords: Cys-loop receptor structure; benzodiazepine; bicuculline; cryo-EM structure; general anesthetic; picrotoxin.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Interests No interests are declared.
Figures


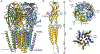
References
-
- Nemecz A, et al. , Emerging Molecular Mechanisms of Signal Transduction in Pentameric Ligand-Gated Ion Channels. Neuron, 2016. 90(3): p. 452–70. - PubMed
-
- Sieghart W and Savic MM, International Union of Basic and Clinical Pharmacology. CVI: GABAA Receptor Subtype- and Function-selective Ligands: Key Issues in Translation to Humans. Pharmacol Rev, 2018. 70(4): p. 836–878. - PubMed
-
- Sigel E and Ernst M, The Benzodiazepine Binding Sites of GABAA Receptors. Trends Pharmacol Sci, 2018. 39(7): p. 659–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

