Portable RT-PCR System: a Rapid and Scalable Diagnostic Tool for COVID-19 Testing
- PMID: 33674285
- PMCID: PMC8091859
- DOI: 10.1128/JCM.03004-20
Portable RT-PCR System: a Rapid and Scalable Diagnostic Tool for COVID-19 Testing
Abstract
Combating the ongoing coronavirus disease 2019 (COVID-19) pandemic demands accurate, rapid, and point-of-care testing with fast results to triage cases for isolation and treatment. The current testing relies on reverse transcriptase PCR (RT-PCR), which is routinely performed in well-equipped laboratories by trained professionals at specific locations. However, during busy periods, high numbers of samples queued for testing can delay the test results, impacting efforts to reduce the infection risk. Besides, the absence of well-established laboratories at remote sites and low-resourced environments can contribute to a silent spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). These reasons compel the need to accommodate point-of-care testing for COVID-19 that meets the ASSURED criteria (
Keywords: Biomeme; COVID-19; RT-PCR; point-of-care; portable.
Copyright © 2021 American Society for Microbiology.
Figures
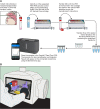
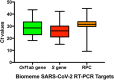
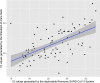
References
-
- World Health Organization. 2020. WHO Director-General's opening remarks at the media briefing on COVID-19 - 16 March 2020. https://www.who.int/director-general/speeches/detail/who-director-genera....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

