A modular approach toward producing nanotherapeutics targeting the innate immune system
- PMID: 33674313
- PMCID: PMC7935355
- DOI: 10.1126/sciadv.abe7853
A modular approach toward producing nanotherapeutics targeting the innate immune system
Abstract
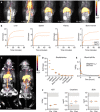
Immunotherapies controlling the adaptive immune system are firmly established, but regulating the innate immune system remains much less explored. The intrinsic interactions between nanoparticles and phagocytic myeloid cells make these materials especially suited for engaging the innate immune system. However, developing nanotherapeutics is an elaborate process. Here, we demonstrate a modular approach that facilitates efficiently incorporating a broad variety of drugs in a nanobiologic platform. Using a microfluidic formulation strategy, we produced apolipoprotein A1-based nanobiologics with favorable innate immune system-engaging properties as evaluated by in vivo screening. Subsequently, rapamycin and three small-molecule inhibitors were derivatized with lipophilic promoieties, ensuring their seamless incorporation and efficient retention in nanobiologics. A short regimen of intravenously administered rapamycin-loaded nanobiologics (mTORi-NBs) significantly prolonged allograft survival in a heart transplantation mouse model. Last, we studied mTORi-NB biodistribution in nonhuman primates by PET/MR imaging and evaluated its safety, paving the way for clinical translation.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




References
-
- Braza M. S., van Leent M. M. T., Lameijer M., Sanchez-Gaytan B. L., Arts R. J. W., Pérez-Medina C., Conde P., Garcia M. R., Gonzalez-Perez M., Brahmachary M., Fay F., Kluza E., Kossatz S., Dress R. J., Salem F., Rialdi A., Reiner T., Boros P., Strijkers G. J., Calcagno C. C., Ginhoux F., Marazzi I., Lutgens E., Nicolaes G. A. F., Weber C., Swirski F. K., Nahrendorf M., Fisher E. A., Duivenvoorden R., Fayad Z. A., Netea M. G., Mulder W. J. M., Ochando J., Inhibiting inflammation with myeloid cell-specific nanobiologics promotes organ transplant acceptance. Immunity 49, 819–828.e6 (2018). - PMC - PubMed
-
- Saeed S., Quintin J., Kerstens H. H. D., Rao N. A., Aghajanirefah A., Matarese F., Cheng S.-C., Ratter J., Berentsen K., van der Ent M. A., Sharifi N., Janssen-Megens E. M., Huurne M. T., Mandoli A., van Schaik T., Ng A., Burden F., Downes K., Frontini M., Kumar V., Giamarellos-Bourboulis E. J., Ouwehand W. H., van der Meer J. W. M., Joosten L. A. B., Wijmenga C., Martens J. H. A., Xavier R. J., Logie C., Netea M. G., Stunnenberg H. G., Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345, 1251086 (2014). - PMC - PubMed
-
- Zhao Y., Fay F., Hak S., Perez-Aguilar J. M., Sanchez-Gaytan B. L., Goode B., Duivenvoorden R., de Lange Davies C., Bjørkøy A., Weinstein H., Fayad Z. A., Pérez-Medina C., Mulder W. J. M., Augmenting drug–carrier compatibility improves tumour nanotherapy efficacy. Nat. Commun. 7, 11221 (2016). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

