The ChAT-acetylcholine pathway promotes group 2 innate lymphoid cell responses and anti-helminth immunity
- PMID: 33674322
- PMCID: PMC8577047
- DOI: 10.1126/sciimmunol.abe3218
The ChAT-acetylcholine pathway promotes group 2 innate lymphoid cell responses and anti-helminth immunity
Abstract
Group 2 innate lymphoid cells (ILC2s) reside in multiple tissues, including lymphoid organs and barrier surfaces, and secrete type 2 cytokines including interleukin-5 (IL-5), IL-9, and IL-13. These cells participate in multiple physiological processes including allergic inflammation, tissue repair, metabolic homeostasis, and host defense against helminth infections. Recent studies indicate that neurotransmitters and neuropeptides can play an important role in regulating ILC2 responses; however, the mechanisms that underlie these processes in vivo remain incompletely defined. Here, we identify that activated ILC2s up-regulate choline acetyltransferase (ChAT)-the enzyme responsible for the biosynthesis of acetylcholine (ACh)-after infection with the helminth parasite Nippostrongylus brasiliensis or treatment with alarmins or cytokines including IL-25, IL-33, and thymic stromal lymphopoietin (TSLP). ILC2s also express acetylcholine receptors (AChRs), and ACh administration promotes ILC2 cytokine production and elicits expulsion of helminth infection. In accordance with this, ChAT deficiency in ILC2s leads to defective ILC2 responses and impaired immunity against helminth infection. Together, these results reveal a previously unrecognized role of the ChAT-ACh pathway in promoting type 2 innate immunity to helminth infection.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
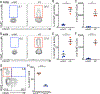
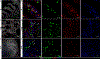

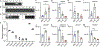




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

