Effects of Gastric Bypass Surgery on the Brain: Simultaneous Assessment of Glucose Uptake, Blood Flow, Neural Activity, and Cognitive Function During Normo- and Hypoglycemia
- PMID: 33674408
- PMCID: PMC8275889
- DOI: 10.2337/db20-1172
Effects of Gastric Bypass Surgery on the Brain: Simultaneous Assessment of Glucose Uptake, Blood Flow, Neural Activity, and Cognitive Function During Normo- and Hypoglycemia
Abstract
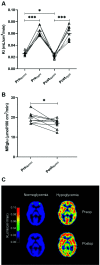
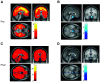
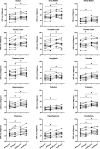
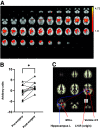
While Roux-en-Y gastric bypass (RYGB) surgery in obese individuals typically improves glycemic control and prevents diabetes, it also frequently causes asymptomatic hypoglycemia. Previous work showed attenuated counterregulatory responses following RYGB. The underlying mechanisms as well as the clinical consequences are unclear. In this study, 11 subjects without diabetes with severe obesity were investigated pre- and post-RYGB during hyperinsulinemic normo-hypoglycemic clamps. Assessments were made of hormones, cognitive function, cerebral blood flow by arterial spin labeling, brain glucose metabolism by 18F-fluorodeoxyglucose (FDG) positron emission tomography, and activation of brain networks by functional MRI. Post- versus presurgery, we found a general increase of cerebral blood flow but a decrease of total brain FDG uptake during normoglycemia. During hypoglycemia, there was a marked increase in total brain FDG uptake, and this was similar for post- and presurgery, whereas hypothalamic FDG uptake was reduced during hypoglycemia. During hypoglycemia, attenuated responses of counterregulatory hormones and improvements in cognitive function were seen postsurgery. In early hypoglycemia, there was increased activation post- versus presurgery of neural networks in brain regions implicated in glucose regulation, such as the thalamus and hypothalamus. The results suggest adaptive responses of the brain that contribute to lowering of glycemia following RYGB, and the underlying mechanisms should be further elucidated.
© 2021 by the American Diabetes Association.
Figures
Comment in
-
Is Bariatric Surgery Brain Surgery?Diabetes. 2021 Jun;70(6):1244-1246. doi: 10.2337/dbi21-0022. Epub 2021 May 20. Diabetes. 2021. PMID: 34016595 Free PMC article. No abstract available.
References
-
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256.e5 - PubMed
-
- Sjöström L, Lindroos A-K, Peltonen M, et al.; Swedish Obese Subjects Study Scientific Group . Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 - PubMed
-
- Morínigo R, Moizé V, Musri M, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 2006;91:1735–1740 - PubMed
-
- Abrahamsson N, Edén Engström B, Sundbom M, Karlsson FA. Hypoglycemia in everyday life after gastric bypass and duodenal switch. Eur J Endocrinol 2015;173:91–100 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

