Proteomics of REPLICANT perfusate detects changes in the metastatic lymph node microenvironment
- PMID: 33674617
- PMCID: PMC7935848
- DOI: 10.1038/s41523-021-00227-7
Proteomics of REPLICANT perfusate detects changes in the metastatic lymph node microenvironment
Abstract
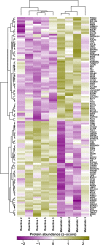
In breast cancer (BC), detecting low volumes of axillary lymph node (ALN) metastasis pre-operatively is difficult and novel biomarkers are needed. We recently showed that patient-derived ALNs can be sustained ex-vivo using normothermic perfusion. We now compare reactive (tumour-free; n = 5) and macrometastatic (containing tumour deposits >2 mm; n = 4) ALNs by combining whole section multiplex immunofluorescence with TMT-labelled LC-MS/MS of the circulating perfusate. Macrometastases contained significantly fewer B cells and T cells (CD4+/CD8+/regulatory) than reactive nodes (p = 0.02). Similarly, pathway analysis of the perfusate proteome (119/1453 proteins significantly differentially expressed) showed that immune function was diminished in macrometastases in favour of 'extracellular matrix degradation'; only 'neutrophil degranulation' was preserved. Qualitative comparison of the perfusate proteome to that of node-positive pancreatic and prostatic adenocarcinoma also highlighted 'neutrophil degranulation' as a contributing factor to nodal metastasis. Thus, metastasis-induced changes in the REPLICANT perfusate proteome are detectable, and could facilitate biomarker discovery.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Giuliano AE, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann. Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

