Random and combinatorial mutagenesis for improved total production of secretory target protein in Escherichia coli
- PMID: 33674702
- PMCID: PMC7935960
- DOI: 10.1038/s41598-021-84859-6
Random and combinatorial mutagenesis for improved total production of secretory target protein in Escherichia coli
Abstract
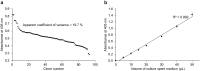
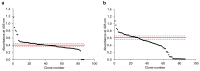
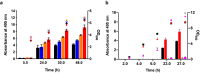
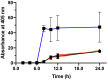
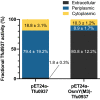
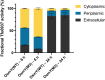
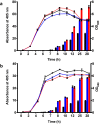
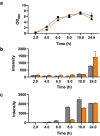
Signal peptides and secretory carrier proteins are commonly used to secrete heterologous recombinant protein in Gram-negative bacteria. The Escherichia coli osmotically-inducible protein Y (OsmY) is a carrier protein that secretes a target protein extracellularly, and we have previously applied it in the Bacterial Extracellular Protein Secretion System (BENNY) to accelerate directed evolution. In this study, we reported the first application of random and combinatorial mutagenesis on a carrier protein to enhance total secretory target protein production. After one round of random mutagenesis followed by combining the mutations found, OsmY(M3) (L6P, V43A, S154R, V191E) was identified as the best carrier protein. OsmY(M3) produced 3.1 ± 0.3 fold and 2.9 ± 0.8 fold more secretory Tfu0937 β-glucosidase than its wildtype counterpart in E. coli strains BL21(DE3) and C41(DE3), respectively. OsmY(M3) also produced more secretory Tfu0937 at different cultivation temperatures (37 °C, 30 °C and 25 °C) compared to the wildtype. Subcellular fractionation of the expressed protein confirmed the essential role of OsmY in protein secretion. Up to 80.8 ± 12.2% of total soluble protein was secreted after 15 h of cultivation. When fused to a red fluorescent protein or a lipase from Bacillus subtillis, OsmY(M3) also produced more secretory protein compared to the wildtype. In this study, OsmY(M3) variant improved the extracellular production of three proteins originating from diverse organisms and with diverse properties, clearly demonstrating its wide-ranging applications. The use of random and combinatorial mutagenesis on the carrier protein demonstrated in this work can also be further extended to evolve other signal peptides or carrier proteins for secretory protein production in E. coli.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Functional production of a soluble and secreted single-chain antibody by a bacterial secretion system.PLoS One. 2014 May 13;9(5):e97367. doi: 10.1371/journal.pone.0097367. eCollection 2014. PLoS One. 2014. PMID: 24824752 Free PMC article.
-
A secretory system for bacterial production of high-profile protein targets.Protein Sci. 2011 Mar;20(3):597-609. doi: 10.1002/pro.593. Protein Sci. 2011. PMID: 21308845 Free PMC article.
-
Proteome-based identification of fusion partner for high-level extracellular production of recombinant proteins in Escherichia coli.Biotechnol Bioeng. 2008 Oct 15;101(3):587-601. doi: 10.1002/bit.21898. Biotechnol Bioeng. 2008. PMID: 18727129
-
Signal peptides for recombinant protein secretion in bacterial expression systems.Microb Cell Fact. 2018 Mar 29;17(1):52. doi: 10.1186/s12934-018-0901-3. Microb Cell Fact. 2018. PMID: 29598818 Free PMC article. Review.
-
Secretory production of recombinant proteins in Escherichia coli.Recent Pat Biotechnol. 2010 Jan;4(1):23-9. doi: 10.2174/187220810790069550. Recent Pat Biotechnol. 2010. PMID: 20201800 Review.
Cited by
-
Extracellular Expression of Feruloyl Esterase and Xylanase in Escherichia coli for Ferulic Acid Production from Agricultural Residues.Microorganisms. 2023 Jul 25;11(8):1869. doi: 10.3390/microorganisms11081869. Microorganisms. 2023. PMID: 37630429 Free PMC article.
-
An iterative strategy to design 4-1BB agonist nanobodies de novo with generative AI models.Sci Rep. 2025 Jul 14;15(1):25412. doi: 10.1038/s41598-025-10241-5. Sci Rep. 2025. PMID: 40659740 Free PMC article.
-
Modes of therapeutic delivery in synthetic microbiology.Trends Microbiol. 2023 Feb;31(2):197-211. doi: 10.1016/j.tim.2022.09.003. Epub 2022 Oct 8. Trends Microbiol. 2023. PMID: 36220750 Free PMC article. Review.
-
Engineering Microbial Consortia as Living Materials: Advances and Prospectives.ACS Synth Biol. 2024 Sep 20;13(9):2653-2666. doi: 10.1021/acssynbio.4c00313. Epub 2024 Aug 22. ACS Synth Biol. 2024. PMID: 39174016 Free PMC article. Review.
-
Advancements in Escherichia coli secretion systems for enhanced recombinant protein production.World J Microbiol Biotechnol. 2025 Mar 3;41(3):90. doi: 10.1007/s11274-025-04302-0. World J Microbiol Biotechnol. 2025. PMID: 40025370 Review.
References
-
- Brockmeier U, Caspers M, Freudl R, Jockwer A, Noll T, Eggert T. Systematic screening of all signal peptides from Bacillus subtilis: A powerful strategy in optimizing heterologous protein secretion in Gram-positive bacteria. J. Mol. Biol. 2006;362(3):393–402. doi: 10.1016/j.jmb.2006.07.034. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

