Evaluation of plasma tau and neurofilament light chain biomarkers in a 12-year clinical cohort of human prion diseases
- PMID: 33674752
- PMCID: PMC8758487
- DOI: 10.1038/s41380-021-01045-w
Evaluation of plasma tau and neurofilament light chain biomarkers in a 12-year clinical cohort of human prion diseases
Abstract
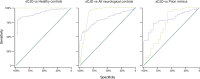
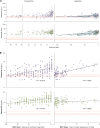
Prion diseases are fatal neurodegenerative conditions with highly accurate CSF and imaging diagnostic tests, but major unmet needs for blood biomarkers. Using ultrasensitive immuno-assays, we measured tau and neurofilament light chain (NfL) protein concentrations in 709 plasma samples taken from 377 individuals with prion disease during a 12 year prospective clinical study, alongside healthy and neurological control groups. This provides an unprecedented opportunity to evaluate their potential as biomarkers. Plasma tau and NfL were increased across all prion disease types. For distinguishing sCJD from control groups including clinically-relevant "CJD mimics", both show considerable diagnostic value. In sCJD, NfL was substantially elevated in every sample tested, including during early disease with minimal functional impairment and in all follow-up samples. Plasma tau was independently associated with rate of clinical progression in sCJD, while plasma NfL showed independent association with severity of functional impairment. In asymptomatic PRNP mutation carriers, plasma NfL was higher on average in samples taken within 2 years of symptom onset than in samples taken earlier. We present biomarker trajectories for nine mutation carriers healthy at enrolment who developed symptoms during follow-up. NfL started to rise as early as 2 years before onset in those with mutations typically associated with more slowly progressive clinical disease. This shows potential for plasma NfL as a "proximity marker", but further work is needed to establish predictive value on an individual basis, and how this varies across different PRNP mutations. We conclude that plasma tau and NfL have potential to fill key unmet needs for biomarkers in prion disease: as a secondary outcome for clinical trials (NfL and tau); for predicting onset in at-risk individuals (NfL); and as an accessible test for earlier identification of patients that may have CJD and require more definitive tests (NfL). Further studies should evaluate their performance directly in these specific roles.
© 2021. The Author(s).
Conflict of interest statement
JC is a Director and JC, JDFW and GSJ are shareholders of D-Gen Limited, an academic spin-out company working in the field of prion disease diagnosis, decontamination and therapeutics. HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).
Figures


References
-
- Surveillance Data from the UK. https://www.cjd.ed.ac.uk/sites/default/files/figs.pdf, 2018, Accessed Date Accessed 2018 Accessed.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

