The gut mycobiome of healthy mice is shaped by the environment and correlates with metabolic outcomes in response to diet
- PMID: 33674757
- PMCID: PMC7935979
- DOI: 10.1038/s42003-021-01820-z
The gut mycobiome of healthy mice is shaped by the environment and correlates with metabolic outcomes in response to diet
Abstract
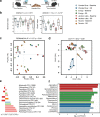
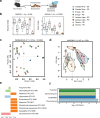
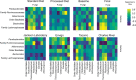
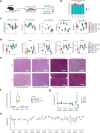
As an active interface between the host and their diet, the gut microbiota influences host metabolic adaptation; however, the contributions of fungi have been overlooked. Here, we investigate whether variations in gut mycobiome abundance and composition correlate with key features of host metabolism. We obtained animals from four commercial sources in parallel to test if differing starting mycobiomes can shape host adaptation in response to processed diets. We show that the gut mycobiome of healthy mice is shaped by the environment, including diet, and significantly correlates with metabolic outcomes. We demonstrate that exposure to processed diet leads to persistent differences in fungal communities that significantly associate with differential deposition of body mass in male mice compared to mice fed standardized diet. Fat deposition in the liver, transcriptional adaptation of metabolically active tissues and serum metabolic biomarker levels are linked with alterations in fungal community diversity and composition. Specifically, variation in fungi from the genera Thermomyces and Saccharomyces most strongly associate with metabolic disturbance and weight gain. These data suggest that host-microbe metabolic interactions may be influenced by variability in the mycobiome. This work highlights the potential significance of the gut mycobiome in health and has implications for human and experimental metabolic studies.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
A focus on fungi in the mouse gut.Lab Anim (NY). 2021 May;50(5):124. doi: 10.1038/s41684-021-00764-5. Lab Anim (NY). 2021. PMID: 33903758 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

