Clinical and functional results after implantation of the bonebridge, a semi-implantable, active transcutaneous bone conduction device, in children and adults
- PMID: 33674927
- PMCID: PMC8738362
- DOI: 10.1007/s00405-021-06626-7
Clinical and functional results after implantation of the bonebridge, a semi-implantable, active transcutaneous bone conduction device, in children and adults
Abstract
Purpose: Aim of the study was to evaluate the surgical, clinical and audiological outcome of 32 implantations of the Bonebridge, a semi-implantable transcutaneous active bone conduction implant.
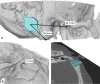
Methods: In a retrospective cohort study, we analyzed data for 32 implantations in 31 patients (one bilateral case; seven age < 16 years) with conductive or mixed hearing loss, malformations, after multiple ear surgery, or with single-sided deafness as contralateral routing of signal (CROS).
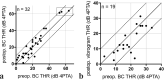
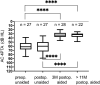
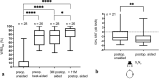
Results: Four implantations were done as CROS. Five cases were simultaneously planned with ear prosthesis anchors, and 23 implantations (72%) were planned through three-dimensional (3D) "virtual surgery." In all 3D-planned cases, the implant could be placed as expected. For implant-related complications, rates were 12.5% for minor and 3.1% for major complications. Implantation significantly improved mean sound field thresholds from a preoperative 60 dB HL (SD 12) to 33 dB HL (SD 6) at 3 postoperative months and 34 dB HL (SD 6) at > 11 postoperative months (p < 0.0001). Word recognition score in quiet at 65 dB SPL improved from 11% (SD 20) preoperatively to 74% (SD 19) at 3 months and 83% (SD 15) at > 11 months (p < 0.0001). The speech reception threshold in noise improved from - 1.01 dB unaided to - 2.69 dB best-aided (p = 0.0018).
Conclusion: We found a clinically relevant audiological benefit with Bonebridge. To overcome anatomical challenges, we recommend preoperative 3D planning in small and hypoplastic mastoids, children, ear malformation, and simultaneous implantation of ear prosthesis anchors and after multiple ear surgery.
Keywords: Bone conduction implant; Bone-anchored hearing aid; Conductive hearing loss; Mixed hearing loss; Transcutaneous hearing implant.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest related to the submitted work. Outside the submitted work the following financial activities are declared for all authors (initials):
Figures
Similar articles
-
Implantable Devices for Single-Sided Deafness and Conductive or Mixed Hearing Loss: A Health Technology Assessment.Ont Health Technol Assess Ser. 2020 Mar 6;20(1):1-165. eCollection 2020. Ont Health Technol Assess Ser. 2020. PMID: 32194878 Free PMC article.
-
Multicentric study on surgical information and early safety and performance results with the Bonebridge BCI 602: an active transcutaneous bone conduction hearing implant.Eur Arch Otorhinolaryngol. 2023 Apr;280(4):1565-1579. doi: 10.1007/s00405-022-07792-y. Epub 2023 Jan 10. Eur Arch Otorhinolaryngol. 2023. PMID: 36625869 Free PMC article. Review.
-
Indication criteria and outcomes with the Bonebridge transcutaneous bone-conduction implant.Laryngoscope. 2014 Dec;124(12):2802-6. doi: 10.1002/lary.24832. Epub 2014 Aug 20. Laryngoscope. 2014. PMID: 25142577
-
A New Active Osseointegrated Implant System in Patients with Single-Sided Deafness.Audiol Neurootol. 2022;27(1):83-92. doi: 10.1159/000515489. Epub 2021 Apr 26. Audiol Neurootol. 2022. PMID: 33902037
-
Long-Term, Multicenter Results With the First Transcutaneous Bone Conduction Implant.Otol Neurotol. 2021 Jul 1;42(6):858-866. doi: 10.1097/MAO.0000000000003159. Otol Neurotol. 2021. PMID: 33989254
Cited by
-
Complications of Transcutaneous Protheses - A Systematic Review of Publications Over the Past 10 Years.Int Arch Otorhinolaryngol. 2022 Feb 4;26(3):e505-e512. doi: 10.1055/s-0042-1742352. eCollection 2022 Jul. Int Arch Otorhinolaryngol. 2022. PMID: 35846823 Free PMC article.
-
Two Bonebridge bone conduction hearing implant generations: audiological benefit and quality of hearing in children.Eur Arch Otorhinolaryngol. 2022 Jul;279(7):3387-3398. doi: 10.1007/s00405-021-07068-x. Epub 2021 Sep 8. Eur Arch Otorhinolaryngol. 2022. PMID: 34495351 Free PMC article.
-
Patients' Experiences of an Active Transcutaneous Implant: The Bone Conduction Implant.Audiol Neurootol. 2025;30(4):335-346. doi: 10.1159/000544774. Epub 2025 Feb 18. Audiol Neurootol. 2025. PMID: 39965558 Free PMC article.
-
Directional sensitivity of bone conduction stimulation on the otic capsule in a finite element model of the human temporal bone.Sci Rep. 2024 Jun 14;14(1):13768. doi: 10.1038/s41598-024-64377-x. Sci Rep. 2024. PMID: 38877090 Free PMC article.
-
Role of early hearing aid experience in speech recognition in patients with bilateral congenital microtia following Bonebridge implantation: a retrospective cohort study.Eur Arch Otorhinolaryngol. 2024 Mar;281(3):1205-1214. doi: 10.1007/s00405-023-08210-7. Epub 2023 Oct 4. Eur Arch Otorhinolaryngol. 2024. PMID: 37792216
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

