Janus kinase signaling as risk factor and therapeutic target for severe SARS-CoV-2 infection
- PMID: 33675065
- PMCID: PMC8250126
- DOI: 10.1002/eji.202149173
Janus kinase signaling as risk factor and therapeutic target for severe SARS-CoV-2 infection
Abstract
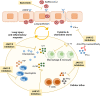
Cytokine signaling, especially interferon (IFN) signaling is closely linked to several aspects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. During initial SARS-CoV-2 infection, symptomatic patients present with impaired type I/III IFN-mediated antiviral responses. Interestingly, IFNs regulate the cellular entry receptor for SARS-CoV-2 on epithelial and endothelial cells. As reported recently, critically ill COVID-19 patients show genetic polymorphisms in one IFN receptor gene (IFNRA2) and in a gene locus near the Janus kinase (JAK) TYK2, which is key for IFN, interleukin (IL)-12 and IL-23 signaling, and T helper (Th) 1/Th17 cell-mediated antiviral immune responses. In the advanced stage of the disease, critically ill COVID-19 patients develop a cytokine storm where many inflammatory mediators using the JAK/STAT signaling pathway such as IL-6, IFN-γ, the granulocyte colony-stimulating factor (G-CSF) or IL-2, and chemokines result in an influx of macrophages and neutrophils damaging the lung tissue. The knowledge on the cytokine and JAK/STAT signaling pathways in severe COVID-19 disease explains the promising first results with JAK inhibitors like baricitinib, which not only dampen the inflammation but in the case of baricitinib also affect virus replication and endocytosis in target cells. Here, we summarize the current immunological associations of SARS-CoV-2 infection with cytokine signaling, the JAK/STAT pathway, and the current clinical stage of JAK inhibitors for improving severe COVID-19 disease.
Keywords: JAK inhibitors; Janus kinase; SARS-CoV-2; cytokine storm; severe COVID-19.
© 2021 The Authors. European Journal of Immunology published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures
References
-
- Galani, I. E. , Rovina, N. , Lampropoulou, V. , Triantafyllia, V. , Manioudaki, M. , Pavlos, E. , Koukaki, E. et al., Untuned antiviral immunity in COVID‐19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2021. 22: 32–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

