Performance evaluation of a lateral flow assay for nasopharyngeal antigen detection for SARS-CoV-2 diagnosis
- PMID: 33675086
- PMCID: PMC8128319
- DOI: 10.1002/jcla.23745
Performance evaluation of a lateral flow assay for nasopharyngeal antigen detection for SARS-CoV-2 diagnosis
Abstract
Background: SARS-CoV-2 has become a global pandemic due to its capacity for rapid transmission. In this context, an early and rapid diagnosis of infected patients that do not require expensive equipment or highly trained personnel is crucial in order to reduce the contagious rate. The aim of this study was to evaluate a chromatographic immunoassay's performance for the rapid diagnosis of SARS-CoV-antigen.
Methods: A cross-sectional study included 369 adults from Western México with diagnosis or suspicion of SARS-CoV-2 infection. Two samples were collected; a naso-oropharyngeal was used for a molecular determination of SARS-CoV-2 RNA. The molecular analysis was carried out using DeCoV19 Kit Triplex (Genes2life S.A.P.I.) based on the CDC diagnostic panel for N1, N2, and N3 regions. The second sample was retrieved from a nasopharyngeal rub and used for the rapid diagnosis of SARS-CoV-2 antigen employing the commercial STANDARD™ Q COVID-19 Ag Test (SD BIOSENSOR).
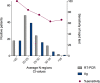
Results: Overall, in 28.2% of the patients was detected the SARS-CoV-2 RNA, and 21.4% were positive for antigen detection. The rapid antigen test showed a sensitivity and specificity of 75.9% and 100%, respectively, with a positive predictive and negative values of 100% and 91%. Symptoms as anosmia presented a high OR for the positive diagnosis for both test, reverse transcription-polymerase chain reaction (RT-PCR), and the rapid antigen test of 8.86 (CI = 4.91-16) and 6.09 (CI = 3.42-10.85), respectively.
Conclusion: SD BIOSENSOR is a useful assay, but some caveats must be considered before the general implementation.
Keywords: COVID-19; SARS-CoV-2; rapid test.
© 2021 The Authors. Journal of Clinical Laboratory Analysis published by Wiley Periodicals LLC.
Conflict of interest statement
Bustillo‐Armendaríz Gustavo and García‐Cedillo Fernanda declared that the rapid antigen test was supplied by P.M.I 12.10, S.A.P.I. de C.V company.
Figures

References
-
- Johns Hopkins University . COVID‐19 Map. Johns Hopkins Coronavirus Resource Center. [cited November 30, 2020]. Available in: https://coronavirus.jhu.edu/map.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

