Implication of TrkC-miR2 in neurotrophin signalling pathway regulation through NGFR transcript targeting
- PMID: 33675128
- PMCID: PMC8034437
- DOI: 10.1111/jcmm.16415
Implication of TrkC-miR2 in neurotrophin signalling pathway regulation through NGFR transcript targeting
Abstract
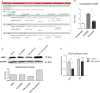
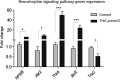
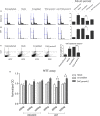
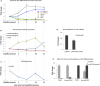
TrkC and NGFR neurotrophin receptors are associated with cell death, cancer and differentiation. TrkC-miR2, which is located in TrkC gene, is known to regulate Wnt signalling pathway, and its influence on other signalling pathways is under investigation. Here, through RT-qPCR, dual-luciferase assay and Western blotting we reveal that TrkC-miR2 targets NGFR. Overexpression of TrkC-miR2 also affected TrkA, TrkC, NFKB, BCL2 and Akt2 expressions involved in neurotrophin signalling pathway, and elevated survival rate of HEK293t and U87 cells was distinguished by flow cytometry and MTT assay. Consistently, an opposite expression correlation was obtained between TrkC-miR2 and NGFR or TrkC for the duration of NT2 differentiation. Meanwhile, TrkC-miR2 down-regulation attenuated NT2 differentiation into neural-like cells. Overall, here we present in silico and experimental evidence showing TrkC-miR2 as a new controller in regulation of neurotrophin signalling pathway.
Keywords: NGFR; TrkC-miR2; neurotrophin signalling pathway.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Figures

Similar articles
-
Overexpression of hsa-miR-939 follows by NGFR down-regulation and apoptosis reduction.J Biosci. 2017 Mar;42(1):23-30. doi: 10.1007/s12038-017-9669-6. J Biosci. 2017. PMID: 28229962
-
A novel microRNA located in the TrkC gene regulates the Wnt signaling pathway and is differentially expressed in colorectal cancer specimens.J Biol Chem. 2017 May 5;292(18):7566-7577. doi: 10.1074/jbc.M116.760710. Epub 2017 Jan 18. J Biol Chem. 2017. PMID: 28100780 Free PMC article.
-
TrkC-miR2 regulates TGFβ signaling pathway through targeting of SMAD3 transcript.J Cell Biochem. 2019 Feb;120(2):2634-2641. doi: 10.1002/jcb.27572. Epub 2018 Oct 10. J Cell Biochem. 2019. PMID: 30304551
-
Molecular interactions between neurotrophin receptors.Cell Tissue Res. 2001 Aug;305(2):229-38. doi: 10.1007/s004410100378. Cell Tissue Res. 2001. PMID: 11545260 Review.
-
Regulation of neurotrophin expression and activity in the retina.Adv Exp Med Biol. 2008;613:343-9. doi: 10.1007/978-0-387-74904-4_40. Adv Exp Med Biol. 2008. PMID: 18188963 Review. No abstract available.
Cited by
-
Modeling of cancer stem cells and the tumor microenvironment Via NT2/D1 cells to probe pathology and treatment for cancer and beyond.Discov Oncol. 2025 Apr 24;16(1):605. doi: 10.1007/s12672-025-02158-2. Discov Oncol. 2025. PMID: 40272656 Free PMC article. Review.
References
-
- Jin W, Kim GM, Kim MS, et al. TrkC plays an essential role in breast tumor growth and metastasis. Carcinogenesis. 2010;31:1939‐1947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

