A novel hydrogen peroxide evolved CHO host can improve the expression of difficult to express bispecific antibodies
- PMID: 33675232
- PMCID: PMC8252053
- DOI: 10.1002/bit.27744
A novel hydrogen peroxide evolved CHO host can improve the expression of difficult to express bispecific antibodies
Abstract
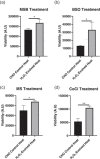
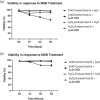
The manufacture of bispecific antibodies by Chinese hamster ovary (CHO) cells is often hindered by lower product yields compared to monoclonal antibodies. Recently, reactive oxygen species have been shown to negatively impact antibody production. By contrast, strategies to boost cellular antioxidant capacity appear to be beneficial for recombinant protein expression. With this in mind, we generated a novel hydrogen peroxide evolved host using directed host cell evolution. Here we demonstrate that this host has heritable resistance to hydrogen peroxide over many generations, displays enhanced antioxidant capacity through the upregulation of several, diverse antioxidant defense genes such as those involved in glutathione synthesis and turnover, and has improved glutathione content. Additionally, we show that this host has significantly improved transfection recovery times, improved growth and viability properties in a fed-batch production process, and elevated expression of two industrially relevant difficult to express bispecific antibodies compared to unevolved CHO control host cells. These findings demonstrate that host cell evolution represents a powerful methodology for improving specific host cell characteristics that can positively impact the expression of difficult to express biotherapeutics.
Keywords: bispecific antibody; evolved host; hydrogen peroxide; redox.
© 2021 AstraZeneca. Biotechnology and Bioengineering published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures





Similar articles
-
Sequence and Configuration of a Novel Bispecific Antibody Format Impacts Its Production Using Chinese Hamster Ovary (CHO) Cells.Biotechnol Bioeng. 2025 Feb;122(2):435-444. doi: 10.1002/bit.28879. Epub 2024 Nov 25. Biotechnol Bioeng. 2025. PMID: 39587782 Free PMC article.
-
miRNA engineering of CHO cells facilitates production of difficult-to-express proteins and increases success in cell line development.Biotechnol Bioeng. 2017 Jul;114(7):1495-1510. doi: 10.1002/bit.26280. Epub 2017 Apr 18. Biotechnol Bioeng. 2017. PMID: 28262952 Free PMC article.
-
Improving product quality and productivity of bispecific molecules through the application of continuous perfusion principles.Biotechnol Prog. 2020 Jul;36(4):e2973. doi: 10.1002/btpr.2973. Epub 2020 Feb 13. Biotechnol Prog. 2020. PMID: 31991523
-
Oxidative stress-alleviating strategies to improve recombinant protein production in CHO cells.Biotechnol Bioeng. 2020 Apr;117(4):1172-1186. doi: 10.1002/bit.27247. Epub 2019 Dec 20. Biotechnol Bioeng. 2020. PMID: 31814104 Free PMC article. Review.
-
Recent advances in CHO cell line development for recombinant protein production.Drug Discov Today Technol. 2020 Dec;38:25-34. doi: 10.1016/j.ddtec.2021.02.003. Epub 2021 Apr 12. Drug Discov Today Technol. 2020. PMID: 34895638 Review.
Cited by
-
Mitochondrial membrane potential-enriched CHO host: a novel and powerful tool for improving biomanufacturing capability.MAbs. 2022 Jan-Dec;14(1):2020081. doi: 10.1080/19420862.2021.2020081. MAbs. 2022. PMID: 35030984 Free PMC article.
-
From Discovery to Delivery: A Rapid and Targeted Proteomics Workflow for Monitoring Chinese Hamster Ovary Biomanufacturing.Mol Cell Proteomics. 2025 Jul;24(7):101011. doi: 10.1016/j.mcpro.2025.101011. Epub 2025 Jun 4. Mol Cell Proteomics. 2025. PMID: 40480342 Free PMC article.
-
Identification of cellular signatures associated with chinese hamster ovary cell adaptation for secretion of antibodies.Comput Struct Biotechnol J. 2024 Dec 10;27:17-31. doi: 10.1016/j.csbj.2024.12.006. eCollection 2025. Comput Struct Biotechnol J. 2024. PMID: 39760073 Free PMC article.
References
-
- Banmeyer, I. , Marchand, C. , Verhaeghe, C. , Vucic, B. , Rees, J. F. , & Knoops, B. (2004). Overexpression of human peroxiredoxin 5 in subcellular compartments of Chinese hamster ovary cells: Effects on cytotoxicity and DNA damage caused by peroxides. Free Radical Biology and Medicine, 36(1), 65–77. 10.1016/j.freeradbiomed.2003.10.019 - DOI - PubMed
-
- Beck, R. , Pedrosa, R. C. , Dejeans, N. , Glorieux, C. , Levêque, P. , Gallez, B. , Taper, H. , Eeckhoudt, S. , Knoops, L. , Calderon, P. B. , & Verrax, J. (2011). Ascorbate/menadione‐induced oxidative stress kills cancer cells that express normal or mutated forms of the oncogenic protein Bcr‐Abl. An in vitro and in vivo mechanistic study. Investigational New Drugs, 29(5), 891–900. 10.1007/s10637-010-9441-3 - DOI - PubMed
-
- Berger, A. , Le Fourn, V. , Masternak, J. , Regamey, A. , Bodenmann, I. , Girod, P. A. , & Mermod, N. (2020). Overexpression of transcription factor Foxa1 and target genes remediate therapeutic protein production bottlenecks in Chinese hamster ovary cells. Biotechnology and Bioengineering, 117(4), 1101–1116. 10.1002/bit.27274 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

