A cell-penetrating CD40-TRAF2,3 blocking peptide diminishes inflammation and neuronal loss after ischemia/reperfusion
- PMID: 33675257
- PMCID: PMC8101361
- DOI: 10.1096/fj.201903203RR
A cell-penetrating CD40-TRAF2,3 blocking peptide diminishes inflammation and neuronal loss after ischemia/reperfusion
Abstract
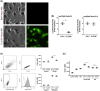
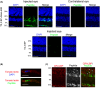
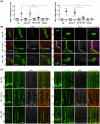
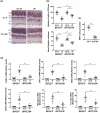
While the administration of anti-CD154 mAbs in mice validated the CD40-CD154 pathway as a target against inflammatory disorders, this approach caused thromboembolism in humans (unrelated to CD40 inhibition) and is expected to predispose to opportunistic infections. There is a need for alternative approaches to inhibit CD40 that avoid these complications. CD40 signals through TRAF2,3 and TRAF6-binding sites. Given that CD40-TRAF6 is the pathway that stimulates responses key for cell-mediated immunity against opportunistic pathogens, we examined the effects of pharmacologic inhibition of CD40-TRAF2,3 signaling. We used a model of ischemia/reperfusion (I/R)-induced retinopathy, a CD40-driven inflammatory disorder. Intravitreal administration of a cell-penetrating CD40-TRAF2,3 blocking peptide impaired ICAM-1 upregulation in retinal endothelial cells and CXCL1 upregulation in endothelial and Müller cells. The peptide reduced leukocyte infiltration, upregulation of NOS2/COX-2/TNF-α/IL-1β, and ameliorated neuronal loss, effects that mimic those observed after I/R in Cd40-/- mice. While a cell-penetrating CD40-TRAF6 blocking peptide also diminished I/R-induced inflammation, this peptide (but not the CD40-TRAF2,3 blocking peptide) impaired control of the opportunistic pathogen Toxoplasma gondii in the retina. Thus, inhibition of the CD40-TRAF2,3 pathway is a novel and potent approach to reduce CD40-induced inflammation, while likely diminishing the risk of opportunistic infections that would otherwise accompany CD40 inhibition.
Keywords: endothelial; muller cell; polymorphonuclear leukocyte; retina; toxoplasma.
© 2021 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
Drs. Carlos S. Subauste and M. Cecilia Subauste have a patent on the CD40‐TRAF2,3 blocking peptide. The other authors have no conflicts of interest to declare.
Figures



References
-
- Boumpas DT, Furie R, Manzi S, et al. A short course of BG9588 (anti‐CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48:719‐727. - PubMed
-
- Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi B. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. - PubMed
-
- Oura T, Yamashita K, Suzuki T, et al. Long‐term hepatic allograft acceptance based on CD40 blockade by ASKP1240 in nonhuman primates. Am J Transplant. 2012;12:1740‐1754. - PubMed
-
- Levy J, Espanol‐Boren T, Thomas C, et al. Clinical spectrum of X‐linked hyper‐IgM syndrome. J Pediatr. 1997;131:47‐54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

