Long-term outcomes of high-dose (74 GyE) proton beam therapy with concurrent chemotherapy for stage III nonsmall-cell lung cancer
- PMID: 33675285
- PMCID: PMC8088926
- DOI: 10.1111/1759-7714.13896
Long-term outcomes of high-dose (74 GyE) proton beam therapy with concurrent chemotherapy for stage III nonsmall-cell lung cancer
Abstract
Background: To evaluate the long-term outcomes of high-dose (74 GyE) proton beam therapy (PBT) with concurrent chemotherapy for stage III non-small cell lung cancer (NSCLC).
Methods: Between July 2007 and March 2018, 45 patients with stage III NSCLC were treated with passive-scattering PBT of 74 GyE and concurrent chemotherapy. Among the 45 patients, the median age was 62 years (range 39-79 years) and 32 patients were men. The clinical stages were stage IIIA in 14 patients and stage IIIB in 31 patients. Thirty-six patients received chemotherapy consisting of cisplatin and vinorelbine.
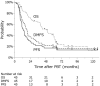
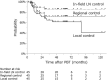
Results: The median follow-up time was 42.1 months (range 6.4-127.0 months) for all patients and 63.5 months (range 9.4-127.0 months) for the 12 survivors. The 3- and 5-year overall survival rates were 63.7% and 38.8%, respectively, and the median overall survival was 49.1 months. Over the follow-up period, disease recurrence was observed in 32 (71%) patients. The 3- and 5-year progression-free survival rates were 22.2% and 17.7%, respectively, with a median progression-free survival of 13.1 months. In-field control improved survival and the in-field control rate was better in patients with T0-3 tumors (p = 0.023) and stage IIIA/IIIB-N3 disease (p = 0.030). Dosimetric parameters of the heart and lung were not associated with survival. No grade 4 or 5 acute or late non-hematologic toxicities were observed.
Conclusions: Passive-scattering PBT of 74 GyE with chemotherapy showed favorable survival and a low incidence of severe adverse events in patients with stage III NSCLC.
Keywords: concurrent chemotherapy; proton beam therapy; stage III non-small cell lung cancer.
© 2021 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures

References
-
- Dillman RO, Herndon J, Seagren SL, Eaton WL Jr, Green MR. Improved survival in stage III non‐small‐cell lung cancer: seven‐year follow‐up of cancer and leukemia group B (CALBG) 8433 trial. J Natl Cancer Inst. 1996;88:1874–81. - PubMed
-
- Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non‐small‐cell lung cancer. J Clin Oncol. 1999;88:1210–5. - PubMed
-
- Perez CA, Stanley K, Rubin P, Kramer S, Brady L, Perez‐Tamayo R, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non‐oat‐cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980;45:2744–53. - PubMed
-
- Bradley JD, Bae K, Graham MV, Byhardt R, Govindan R, Fowler J, et al. Primary analysis of the phase II component of a phase I/II dose intensification study using three‐dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non‐small‐cell lung cancer: RTOG 0117. J Clin Oncol. 2010;28:2475–80. - PMC - PubMed
-
- Bradley JD, Moughan J, Graham MV, Byhardt R, Govindan R, Fowler J, et al. A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non‐small‐cell lung cancer: phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys. 2010;77:367–72. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

