The Transcription Factor Shox2 Shapes Neuron Firing Properties and Suppresses Seizures by Regulation of Key Ion Channels in Thalamocortical Neurons
- PMID: 33675359
- PMCID: PMC8196244
- DOI: 10.1093/cercor/bhaa414
The Transcription Factor Shox2 Shapes Neuron Firing Properties and Suppresses Seizures by Regulation of Key Ion Channels in Thalamocortical Neurons
Abstract
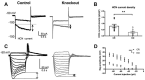
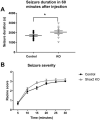
Thalamocortical neurons (TCNs) play a critical role in the maintenance of thalamocortical oscillations, dysregulation of which can result in certain types of seizures. Precise control over firing rates of TCNs is foundational to these oscillations, yet the transcriptional mechanisms that constrain these firing rates remain elusive. We hypothesized that Shox2 is a transcriptional regulator of ion channels important for TCN function and that loss of Shox2 alters firing frequency and activity, ultimately perturbing thalamocortical oscillations into an epilepsy-prone state. In this study, we used RNA sequencing and quantitative PCR of control and Shox2 knockout mice to determine Shox2-affected genes and revealed a network of ion channel genes important for neuronal firing properties. Protein regulation was confirmed by Western blotting, and electrophysiological recordings showed that Shox2 KO impacted the firing properties of a subpopulation of TCNs. Computational modeling showed that disruption of these conductances in a manner similar to Shox2's effects modulated frequency of oscillations and could convert sleep spindles to near spike and wave activity, which are a hallmark for absence epilepsy. Finally, Shox2 KO mice were more susceptible to pilocarpine-induced seizures. Overall, these results reveal Shox2 as a transcription factor important for TCN function in adult mouse thalamus.
Keywords: HCN channel; epilepsy; oscillations; t-type Ca2+ channel; thalamus.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures







Similar articles
-
Modulation of thalamocortical oscillations by TRIP8b, an auxiliary subunit for HCN channels.Brain Struct Funct. 2018 Apr;223(3):1537-1564. doi: 10.1007/s00429-017-1559-z. Epub 2017 Nov 22. Brain Struct Funct. 2018. PMID: 29168010 Free PMC article.
-
Medium-voltage 5-9-Hz oscillations give rise to spike-and-wave discharges in a genetic model of absence epilepsy: in vivo dual extracellular recording of thalamic relay and reticular neurons.Neuroscience. 2001;105(1):181-201. doi: 10.1016/s0306-4522(01)00182-8. Neuroscience. 2001. PMID: 11483311
-
Sleep spindles are generated in the absence of T-type calcium channel-mediated low-threshold burst firing of thalamocortical neurons.Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):20266-71. doi: 10.1073/pnas.1320572110. Epub 2013 Nov 26. Proc Natl Acad Sci U S A. 2013. PMID: 24282303 Free PMC article.
-
T-type Ca2+ channels in absence epilepsy.Pflugers Arch. 2014 Apr;466(4):719-34. doi: 10.1007/s00424-014-1461-y. Epub 2014 Feb 13. Pflugers Arch. 2014. PMID: 24519464 Review.
-
Interactions between membrane conductances underlying thalamocortical slow-wave oscillations.Physiol Rev. 2003 Oct;83(4):1401-53. doi: 10.1152/physrev.00012.2003. Physiol Rev. 2003. PMID: 14506309 Free PMC article. Review.
Cited by
-
Hippocampus RNA Sequencing of Pentylenetetrazole-Kindled Rats and Upon Treatment of Novel Chemical Q808.Front Pharmacol. 2022 Mar 8;13:820508. doi: 10.3389/fphar.2022.820508. eCollection 2022. Front Pharmacol. 2022. PMID: 35345815 Free PMC article.
-
IGF2BP2-Shox2 axis regulates hippocampal-neuronal senescence to alleviate microgravity-induced recognition disturbance.iScience. 2024 May 7;27(6):109917. doi: 10.1016/j.isci.2024.109917. eCollection 2024 Jun 21. iScience. 2024. PMID: 38812544 Free PMC article.
-
A Distinct Down-to-Up Transition Assembly in the Retrosplenial Cortex during Slow-Wave Sleep.J Neurosci. 2025 Apr 2;45(14):e1484242025. doi: 10.1523/JNEUROSCI.1484-24.2025. J Neurosci. 2025. PMID: 39952672
-
AMPK Phosphorylates LMX1b to Regulate a Brainstem Neurogenic Network Important for Control of Breathing in Neonatal Mice.Int J Mol Sci. 2024 Dec 30;26(1):213. doi: 10.3390/ijms26010213. Int J Mol Sci. 2024. PMID: 39796072 Free PMC article.
-
The Contribution of HCN Channelopathies in Different Epileptic Syndromes, Mechanisms, Modulators, and Potential Treatment Targets: A Systematic Review.Front Mol Neurosci. 2022 May 19;15:807202. doi: 10.3389/fnmol.2022.807202. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35663267 Free PMC article.
References
-
- Arcelli P, Frassoni C, Regondi MC, De Biasi S, Spreafico R. 1997. GABAergic neurons in mammalian thalamus: a marker of thalamic complexity? Brain Res Bull. 42:27–37. - PubMed
-
- Avoli M, Gloor P, Kostopoulos G, Gotman J. 1983. An analysis of penicillin-induced generalized spike and wave discharges using simultaneous recordings of cortical and thalamic single neurons. J Neurophysiol. 50:819–837. - PubMed
-
- Bal T, McCormick DA. 1996. What stops synchronized thalamocortical oscillations? Neuron. 17:297–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

