Effects of Er:YAG laser irradiation of different titanium surfaces on osteoblast response
- PMID: 33675441
- PMCID: PMC7936964
- DOI: 10.1007/s10856-021-06493-y
Effects of Er:YAG laser irradiation of different titanium surfaces on osteoblast response
Abstract
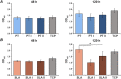
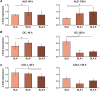
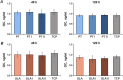
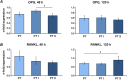
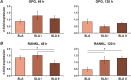
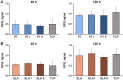
The aim of this in vitro study was to evaluate the effects of erbium-doped yttrium aluminum garnet (Er:YAG) laser irradiation on titanium surface topography and the proliferation and differentiation of osteoblasts using standard clinical treatment settings. Er:YAG laser irradiation at two levels ((1): 160 mJ, pulse at 20 Hz; (2): 80 mJ, pulse at 20 Hz) was applied to moderately rough and smooth titanium disks before MG-63 osteoblast-like cells were cultured on these surfaces. Titanium surface and cell morphology were observed by scanning electron microscopy. Cell proliferation/viability was measured by CCK-8 test. Gene expression of alkaline phosphatase (ALP), osteocalcin (OC), osteoprotegerin (OPG), receptor activator of nuclear factor kappa-B ligand (RANKL), and collagen type 1 was measured by qPCR, and OPG and OC protein production was determined by enzyme-linked immunosorbent assay. Treatment with Er:YAG laser at 160 mJ/20 Hz markedly caused heat-induced fusion of titanium and cell condensation on moderately rough surfaces, but not in smooth surfaces. MG-63 proliferation/viability decreased after 5 days in moderately rough surfaces. The expression of ALP, OC, OPG, and collagen type 1 was unaffected by laser treatment at 160 mJ/20. Laser irradiation at 80 mJ/20 Hz enhanced RANKL gene expression after 5 days in moderately rough surfaces. Study results suggest that Er:YAG laser irradiation at clinically relevant setting has no essential effect on osteogenic gene and protein expression of osteoblasts. However, surface structure, cell attachment, and proliferation are influenced by both treatment protocols, which implies that caution should be taken in the clinical treatment of peri-implant diseases when Er:YAG laser is used.
Keywords: Erbium laser; Osteoblasts; Osteogenesis; Surface decontamination; Titanium surface.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
An in vitro evaluation of the responses of human osteoblast-like SaOs-2 cells to SLA titanium surfaces irradiated by erbium:yttrium-aluminum-garnet (Er:YAG) lasers.Lasers Med Sci. 2014 Jan;29(1):47-53. doi: 10.1007/s10103-012-1224-y. Epub 2012 Nov 20. Lasers Med Sci. 2014. PMID: 23179305
-
Effects of Er: YAG laser irradiation on wettability, surface roughness, and biocompatibility of SLA titanium surfaces: an in vitro study.Lasers Med Sci. 2015 Feb;30(2):561-6. doi: 10.1007/s10103-013-1361-y. Epub 2013 Jun 13. Lasers Med Sci. 2015. PMID: 23760881
-
The effects of Er:YAG laser treatment on titanium surface profile and osteoblastic cell activity: an in vitro study.J Periodontol. 2011 Aug;82(8):1169-77. doi: 10.1902/jop.2010.100428. Epub 2010 Dec 28. J Periodontol. 2011. PMID: 21189088
-
The Effect of Erbium-Doped Yttrium Aluminum Garnet Laser in Debonding of Orthodontic Brackets: A Systematic Review of the Literature.Photobiomodul Photomed Laser Surg. 2021 Nov;39(11):725-733. doi: 10.1089/photob.2020.4985. Epub 2021 May 17. Photobiomodul Photomed Laser Surg. 2021. PMID: 33999734
-
Evaluation of zirconia surface roughness after aluminum oxide airborne-particle abrasion and the erbium-YAG, neodymium-doped YAG, or CO2 lasers: A systematic review and meta-analysis.J Prosthet Dent. 2019 Jun;121(6):895-903.e2. doi: 10.1016/j.prosdent.2018.07.001. Epub 2019 Jan 31. J Prosthet Dent. 2019. PMID: 30711290
Cited by
-
Influence of Clinical Decontamination Techniques on the Surface Characteristics of SLA Titanium Implant.Nanomaterials (Basel). 2022 Dec 18;12(24):4481. doi: 10.3390/nano12244481. Nanomaterials (Basel). 2022. PMID: 36558334 Free PMC article.
-
Efficacy of Er:YAG Laser as a Debridement Method in Surgical Treatment for Peri-Implantitis: A Systematic Review.Oral Health Prev Dent. 2023 Jan 18;21:17-24. doi: 10.3290/j.ohpd.b3818041. Oral Health Prev Dent. 2023. PMID: 36651312 Free PMC article.
-
Effects of hydroxyapatite-coated porous titanium scaffolds functionalized by exosomes on the regeneration and repair of irregular bone.Front Bioeng Biotechnol. 2023 Oct 31;11:1283811. doi: 10.3389/fbioe.2023.1283811. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38026868 Free PMC article.
-
Microbiological and Imaging-Based Evaluations of Photodynamic Therapy Combined with Er:YAG Laser Therapy in the In Vitro Decontamination of Titanium and Zirconia Surfaces.Microorganisms. 2024 Jun 30;12(7):1345. doi: 10.3390/microorganisms12071345. Microorganisms. 2024. PMID: 39065113 Free PMC article.
-
Titanium Surfaces with a Laser-Produced Microchannel Structure Enhance Pre-Osteoblast Proliferation, Maturation, and Extracellular Mineralization In Vitro.Int J Mol Sci. 2024 Mar 16;25(6):3388. doi: 10.3390/ijms25063388. Int J Mol Sci. 2024. PMID: 38542358 Free PMC article.
References
-
- Albrektsson T, Wennerberg A. Oral implant surfaces: part 1-review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004;17:536–43. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

