Strategies to improve retention in randomised trials
- PMID: 33675536
- PMCID: PMC8092429
- DOI: 10.1002/14651858.MR000032.pub3
Strategies to improve retention in randomised trials
Abstract
Background: Poor retention of participants in randomised trials can lead to missing outcome data which can introduce bias and reduce study power, affecting the generalisability, validity and reliability of results. Many strategies are used to improve retention but few have been formally evaluated.
Objectives: To quantify the effect of strategies to improve retention of participants in randomised trials and to investigate if the effect varied by trial setting.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Scopus, PsycINFO, CINAHL, Web of Science Core Collection (SCI-expanded, SSCI, CPSI-S, CPCI-SSH and ESCI) either directly with a specified search strategy or indirectly through the ORRCA database. We also searched the SWAT repository to identify ongoing or recently completed retention trials. We did our most recent searches in January 2020.
Selection criteria: We included eligible randomised or quasi-randomised trials of evaluations of strategies to increase retention that were embedded in 'host' randomised trials from all disease areas and healthcare settings. We excluded studies aiming to increase treatment compliance.
Data collection and analysis: We extracted data on: the retention strategy being evaluated; location of study; host trial setting; method of randomisation; numbers and proportions in each intervention and comparator group. We used a risk difference (RD) and 95% confidence interval (CI) to estimate the effectiveness of the strategies to improve retention. We assessed heterogeneity between trials. We applied GRADE to determine the certainty of the evidence within each comparison.
Main results: We identified 70 eligible papers that reported data from 81 retention trials. We included 69 studies with more than 100,000 participants in the final meta-analyses, of which 67 studies evaluated interventions aimed at trial participants and two evaluated interventions aimed at trial staff involved in retention. All studies were in health care and most aimed to improve postal questionnaire response. Interventions were categorised into broad comparison groups: Data collection; Participants; Sites and site staff; Central study management; and Study design. These intervention groups consisted of 52 comparisons, none of which were supported by high-certainty evidence as determined by GRADE assessment. There were four comparisons presenting moderate-certainty evidence, three supporting retention (self-sampling kits, monetary reward together with reminder or prenotification and giving a pen at recruitment) and one reducing retention (inclusion of a diary with usual follow-up compared to usual follow-up alone). Of the remaining studies, 20 presented GRADE low-certainty evidence and 28 presented very low-certainty evidence. Our findings do provide a priority list for future replication studies, especially with regard to comparisons that currently rely on a single study.
Authors' conclusions: Most of the interventions we identified aimed to improve retention in the form of postal questionnaire response. There were few evaluations of ways to improve participants returning to trial sites for trial follow-up. None of the comparisons are supported by high-certainty evidence. Comparisons in the review where the evidence certainty could be improved with the addition of well-done studies should be the focus for future evaluations.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Two of the review authors (ST and GR) are authors on two of the eligible studies (Bailey 2013; Treweek 2020a). There are no other conflicts to declare.
Figures
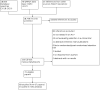

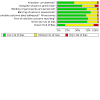
























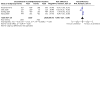






























Comment in
-
Cochrane in CORR: Strategies to Improve Retention in Randomised Trials.Clin Orthop Relat Res. 2022 Jan 1;480(1):23-28. doi: 10.1097/CORR.0000000000002028. Clin Orthop Relat Res. 2022. PMID: 34699387 Free PMC article. No abstract available.
References
References to studies included in this review
AMBER 2020 {unpublished data only}
-
- Hagen S. Evaluation of theory informed cover letetr comapred to standard cover letter. Personal Communication.
Arundel 2019 {published data only}
Ashby 2011 {published data only}
Avenell 2004 {published data only}
-
- Avenell A, Grant AM, McGee M, McPherson G, Campbell MK, McGee MA, et al. The effects of an open design on trial participant recruitment, compliance and retention - a randomized controlled trial comparison with a blinded, placebo-controlled design. Clinical Trials 2004;1(6):490-8. [DOI: 10.1191/1740774504cn053oa] - DOI - PubMed
Bailey 2013 {published data only (unpublished sought but not used)}
-
- Bailey J. Evaluating the effectiveness of incentives in the "Sexunzipped" trial. Personal communication - email.
Bauer 2004 {published data only}
-
- Bauer JE, Rezaishiraz H, Head K, Cowell J, Bepler G, Aiken M, et al. Obtaining DNA from a geographically dispersed cohort of current and former smokers: use of mail-based mouthwash collection and monetary incentives. Nicotine and Tobacco Research 2004;6(3):439-46. [DOI: 10.1080/14622200410001696583] - DOI - PubMed
Bean 2019 {published data only}
Bell 2016 {published data only}
Bradshaw 2020 {published data only}
Brubaker 2019 {published data only}
-
- Brubaker L, Jelovsek JE, Lukacz ES, Balgobin S, Ballard A, Weidner AC, et al. Recruitment and retention: a randomized controlled trial of video-enhanced versus standard consent processes within the E-OPTIMAL study. Clinical Trials 2019;16(5):481-9. [DOI: 10.1177/1740774519865541] - DOI - PMC - PubMed
Clark 2015 {published data only}
-
- Clark L, Ronaldson S, Dyson L, Hewitt C, Torgerson D, Adamson J. Electronic prompts significantly increase response rates to postal questionnaires: a randomized trial within a randomized trial and meta-analysis. Journal of Clinical Epidemiology 2015;68(12):1446-50. [DOI: 10.1016/j.jclinepi.2015.01.016] - DOI - PubMed
Cochrane 2020 {published data only}
-
- Cochrane A, Welch C, Fairhurst C, Cockayne S, Torgerson DJ, OTIS Study Group. An evaluation of a personalised text message reminder compared to a standard text message on postal questionnaire response rates: an embedded randomised controlled trial. F1000Research 2020;9:154. [DOI: 10.12688/f1000research.22361.1] - DOI - PMC - PubMed
Cockayne 2005 {published and unpublished data}
Cockayne 2017 {published data only}
-
- Cockayne S, Fairhurst C, Adamson J, Hewitt C, Hull R, Hicks K, et al. An optimised patient information sheet did not significantly increase recruitment or retention in a falls prevention study: an embedded randomised recruitment trial. Trials 2017;18(1):144. [DOI: 10.1186/s13063-017-1797-7] - DOI - PMC - PubMed
Cook 2020 {unpublished data only}
-
- Cook J, Cook JA, Bongard E, Heneghan C, Butler CC. SWAT 90 evaluation: Does the time at which a participant incentive is given affect the retention rate? https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog.... [16406421]
Couper 2007 {published data only (unpublished sought but not used)}
Cunningham 2004 {published and unpublished data}
Cunningham‐Burley 2020 {published data only}
Dinglas 2015 {published data only}
Dorling 2020 {published data only}
Dorman 1997 {published and unpublished data}
Edwards 2004 {published data only}
Edwards 2016 {published data only}
Fouad 2014 {published data only}
Gates 2009 {published data only}
Gattellari 2004 {published data only}
Glassman 2020 {published data only}
Goulao 2020 {published data only}
Goulao 2020 (replication of SWAT #2) {published data only}
Greig 2017 {published data only}
Griffin 2019 {published data only}
Guarino 2006 {published data only}
Hardy 2016 {published data only}
-
- Hardy P, Bell JL, Brocklehurst P, Epidural and Position Trial Collaborative Group. Evaluation of the effects of an offer of a monetary incentive on the rate of questionnaire return during follow-up of a clinical trial: a randomised study within a trial. BMC Medical Research Methodology 2016;16:82. [DOI: 10.1186/s12874-016-0180-9] - DOI - PMC - PubMed
Henderson 2010 {published and unpublished data}
James 2020 {published data only}
-
- James S, Parker A, Cockayne S, Rodgers S, Fairhurst C, Torgerson D, et al. Including a pen and/or cover letter, containing social incentive text, had no effect on questionnaire response rate: a factorial randomised controlled Study within a Trial. F1000Research 2020;9:623. [DOI: 10.12688/f1000research.23767.1] - DOI - PMC - PubMed
Keding 2016 {published data only}
Kenton 2007 {published data only}
-
- Kenton L, Dennis CL, Weston J, Kiss A. The effect of incentives and high priority mailing on postal questionnaire response rates: a mini-RCT. In: Abstracts from the 28th Meeting of the Society of Clinical Trials; 2007 May 20-23; Montreal. Vol. 4. 2007:371-455.
Kenyon 2005 {published data only}
Khadjesari 2011 {published and unpublished data}
Land 2007 {published and unpublished data}
Lewis 2017 {published data only}
Lienard 2006 {published data only}
-
- Liénard JL, Quinaux E, Fabre-Guillevin E, Piedbois P, Jouhaud A, Decoster G, et al. Impact of on-site initiation visits on patient recruitment and data quality in a randomized trial of adjuvant chemotherapy for breast cancer. Clinical Trials 2006;3(5):486-92. [DOI: 10.1177/1740774506070807] - DOI - PubMed
MacLennan 2014 {published data only}
-
- MacLennan G, McDonald A, McPherson G, Treweek S, Avenell A, RECORD Trial Group. Advance telephone calls ahead of reminder questionnaires increase response rate in non-responders compared to questionnaire reminders only: The RECORD phone trial. Trials 2014;15:13. [DOI: 10.1186/1745-6215-15-13] - DOI - PMC - PubMed
MamMOTH 2020 {unpublished data only}
-
- Beasley M. MamMOTH trial. Personal communication.
Man 2011 {published data only}
Marques 2013 {published data only}
Marsh 1999 {published data only}
Marson 2007 {published and unpublished data}
McCambridge 2011 {published data only}
McColl 2003 ‐ Trial 1 {published and unpublished data}
McColl 2003 ‐ Trial 2 {published and unpublished data}
Mitchell 2011 {published data only}
Mitchell 2012 {published data only}
-
- Mitchell N, Hewitt CE, Lenaghan E, Platt E, Shepstone L, Torgerson DJ, SCOOP study team. Prior notification of trial participants by newsletter increased response rates: a randomized controlled trial. Journal of Clinical Epidemiology 2012;65(12):1348-52. [DOI: 10.1016/j.jclinepi.2012.05.008] - DOI - PubMed
Mitchell 2020a {published and unpublished data}
Mitchell 2020b {published data only}
Nakash 2007 {unpublished data only}
-
- Nakash RA. A Study of Response and Non-Response to Postal Questionnaire Follow-up in Clinical Trials: Chapter 6: A Randomised Controlled Trial of a Method of Improving Response to Postal Questionnaire Follow-up in a Clinical Trial [PhD thesis]. Warwick, UK: University of Warwick, Warwick Medical School, 2007.
OPAL 2020 {unpublished data only}
-
- Goodman K. Evaluation of theory informed cover letter compared to standard cover letter.. Personal communication.
Renfroe 2002 {published and unpublished data}
-
- Renfroe EG, Heywood G, Foreman L, Schron E, Powell J, Baessler C, et al. The end-of-study patient survey: methods influencing response rate in the AVID trial. Controlled Clinical Trials 2002;23(5):521-33. - PubMed
-
- Renfroe EG, Powell J, Olarte A, Morris M, Hallstrom A. Method of administering patient surveys affects response rate. Controlled Clinical Trials 1999;20:s62. [DOI: 10.1016/s0197-2456(02)00225-8] - DOI
Rodgers 2019 {published data only}
-
- Rodgers S, Sbizzera I, Cockayne S, Fairhurst C, Lamb SE, Vernon W, et al. A study update newsletter or Post-it® note did not increase postal questionnaire response rates in a falls prevention trial: an embedded randomised factorial trial. F1000Research 2019;7:1083. [DOI: 10.12688/f1000research.14591.2] - DOI - PMC - PubMed
Salvesen 1992 {published and unpublished data}
Sarathy 2020 {published data only}
-
- Sarathy PP, Kottam L, Parker A, Brealey S, Coleman E, Keding A, et al. Timing of electronic reminders did not improve trial participant questionnaire response: a randomized trial and meta-analyses. Journal of Clinical Epidemiology 2020;6:eprint. - PubMed
Severi 2011 {published data only}
-
- Severi E, Free C, Knight R, Robertson S, Edwards P, Hoile E. Two controlled trials to increase participant retention in a randomized controlled trial of mobile phone-based smoking cessation support in the United Kingdom. Clinical Trials 2011;8(5):654-60. [DOI: 10.1177/1740774511416524] - DOI - PMC - PubMed
Sharp 2006 {published data only}
Starr 2015 {published data only}
Subar 2001 {published data only}
-
- Subar AF, Ziegler RG, Thompson FE, Johnson CC, Weissfeld JL, Reding D, et al. Is shorter always better? Relative importance of questionnaire length and cognitive ease on response rates and data quality for two dietary questionnaires. American Journal of Epidemiology 2001;153(4):404-9. [DOI: 10.1093/aje/153.4.404] - DOI - PubMed
Tai 1997 {published data only}
Tilbrook 2015 {published data only}
-
- Tilbrook HE, Becque T, Buckley H, MacPherson H, Bailey M, Torgerson DJ. Randomized trial within a trial of yellow 'post-it notes' did not improve questionnaire response rates among participants in a trial of treatments for neck pain. Journal of Evaluation in Clinical Practice 2015;21(2):202-4. [DOI: 10.1111/jep.12284] - DOI - PubMed
Tranberg 2018 {published data only}
-
- Tranberg M, Bech BH, Blaakær J, Jensen JS, Svanholm H, Andersen B. Preventing cervical cancer using HPV self-sampling: direct mailing of test-kits increases screening participation more than timely opt-in procedures - a randomized controlled trial. BMC Cancer 2018;18(1):273. [DOI: 10.1186/s12885-018-4165-4] - DOI - PMC - PubMed
Treweek 2020a {unpublished data only}
-
- Treweek S, Gallant S, Anderson AS. SWAT 76 evaluation: randomised evaluation of sending pre-notification cards to trial participants before a face-to-face primary outcome measurement. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog.... - PMC - PubMed
Watson 2017 {published data only}
-
- Watson AJ, Cook J, Hudson J, Kilonzo M, Wood J, Bruhn H, et al. A pragmatic multicentre randomised controlled trial comparing stapled haemorrhoidopexy with traditional excisional surgery for haemorrhoidal disease: the eTHoS study. Health Technology Assessment 2017;21(70):1-224. [DOI: 10.3310/hta21700] - DOI - PMC - PubMed
Whiteside 2019 {published data only}
Young 2020 {published data only}
-
- Young B, Bedford L, das Nair R, Gallant S, Littleford R, Robertson JF, et al. Unconditional and conditional monetary incentives to increase response to mailed questionnaires: A randomized controlled study within a trial (SWAT). Journal of Evaluation in Clinical Practice 2020;26(3):893-902. [DOI: 10.1111/jep.13230] - DOI - PubMed
References to studies excluded from this review
Abboah‐Offei 2020 {published data only}
-
- Abboah-Offei M, Bristowe K, Vanderpuye-Donton NA, Ansa G, Oppong-Agyei YD, Abas M, et al. Phase II mixed methods' feasibility cluster randomised controlled trial of a novel community-based enhanced care intervention to improve person-centred outcomes for people living with HIV in Ghana. AIDS Care 2020;32(sup 2):107-18. [DOI: 10.1080/09540121.2020.1739217] - DOI - PubMed
Alexander 2008 {published data only}
Arnevik 2009 {published data only}
-
- Arnevik E, Wilberg T, Urnes Ø, Johansen M, Monsen J, Karterud S. Psychotherapy for personality disorders: short-term day hospital psychotherapy versus outpatient individual therapy - a randomized controlled study. European Psychiatry 2009;24:71-8. - PubMed
Arundel 2017 {published data only}
-
- Arundel C, Jefferson L, Bailey M, Cockayne S, Hicks K, Loughrey L, et al. A randomized, embedded trial of pre?notification of trial participation did not increase recruitment rates to a falls prevention trial. Journal of Evaluation in Clinical Practice 2017;23(1):73-8. [DOI: 10.1111/jep.12576] - DOI - PubMed
Atherton 2010 {published data only}
-
- Atherton H, Oakeshott P, Aghaizu A, Hay P, Kerry S. Use of an online questionnaire for follow-up of young female students recruited to a randomised controlled trial of chlamydia screening. Journal of Epidemiology and Community Health 2010;64(580):e584. - PubMed
Aysola 2018 {published data only}
Barry 1996 {published and unpublished data}
-
- Barry MJ, Walker-Corkery E, Chang Y, Tyll LT, Cherkin DC, Fowler FJ. Measurement of overall and disease-specific health status: does the order of questionnaires make a difference. Journal of Health Services Research and Policy 1996;1(1):20-7. - PubMed
Bednarek 2008 {published data only}
-
- Bednarek P, Nichols M, Carlson N, Edelman A, Creinin M, Truitt S, et al. Effect of "observed start" vs. traditional "Sunday start" on hormonal contraceptive continuation rates after medical abortion. Contraception 2008;78:26-30. - PubMed
Bisla 2019 {published data only}
-
- Bisla J, Ambler G, Frank B, Gulati S, Hocken P, James M, et al. Successful and unsuccessful recruitment and retainment strategies in a UK multicentre drug trial for a rare chronic pain condition which performed above target. British Journal of Pain 2020;14(3):171-9. [DOI: 10.1177/2049463719893399] - DOI - PMC - PubMed
Bowen 2000 {published and unpublished data}
-
- Bowen D, Thornquist M, Anderson K, Barnett M, Valanis B, Goodman G, et al. The use of incentives to increase participation in a chemoprevention trial. Controlled Clinical Trials 1995;16:41s.
-
- Bowen D, Thornquist M, Goodman G, Omenn GS, Anderson K, Barnett M, et al. Effects of incentive items on participation in a randomized chemoprevention trial. Journal of Health and Psychology 2000;5(1):109-15. - PubMed
Bromley 2019 {published data only}
-
- Bromley KJ, Ogollah R, Konstantinou K, Foster N, Lewis M. The use of regular text messaging over one year to collect primary outcome data in a randomised controlled trial. Trials 2019;20(Suppl 1):12.
Chaffin 2009 {published and unpublished data}
-
- Chaffin M, Valle LA, Funderburk B, Gurwitch R, Silovsky J, Bard D. A motivational intervention can improve retention in PCIT for low-motivation child welfare clients. Child Maltreatment 2009;14(4):356-68. - PubMed
Chee 2019 {published data only}
Cheung 2019 {published data only}
Cox 2003 {published data only}
-
- Cox KL, Burke V, Gorely TJ, Beilin LJ, Puddey IB. Controlled comparison of retention and adherence in home- vs center-initiated exercise interventions in women ages 40?65 years: the S.W.E.A.T. study (Sedentary Women Exercise Adherence Trial). Preventive Medicine 2003;36:17-29. - PubMed
Cox 2006 {published data only}
Cox 2008 {published and unpublished data}
-
- Cox KL, Burke V, Beilin LJ, Derbyshire AJ, Grove JR, Blanksby BA, et al. Short and long-term adherence to swimming and walking programs in older women - the Sedentary Women Exercise Adherence Trial (SWEAT 2). Preventive Medicine 2008;46(6):511-7. - PubMed
Day 1998 {published data only}
-
- Day R, Cella D, Ganz P, Constantino J. Electronic monitoring of participant adherence in the NSABP breast cancer prevention trial (BCPT). Controlled Clinical Trials 1998;19:69s.
Diaz 2001 {published data only}
Eaker 2004 {published data only}
-
- Eaker S, Adami H, Granath F, Wilander E, Sparen P. A large population-based randomized controlled trial to increase attendance at screening for cervical cancer. Cancer Epidemiology, Biomarkers & Prevention 2004;13(3):346-54. - PubMed
Edelstein 2005 {published data only}
-
- Edelstein S, Minutillo A, Pyle L, Anderson B, Wilfley D, Van Buren D, et al. Rewarding youth behaviour: implementation of incentives in the TODAY trial. Clinical Trials 2005;2:s23.
Edwards 2013 {published data only}
-
- Edwards KE, Hagen SM, Hannam J, Kruger C, Yu R, Merry AF. A randomized comparison between records made with an anesthesia information management system and by hand, and evaluation of the Hawthorne effect. Canadian Journal of Anaesthesia 2013;60(10):990-7. [DOI: 10.1007/s12630-013-0003-y] - DOI - PubMed
Farabee 2016 {published data only}
Ford 2006 {published and unpublished data}
-
- Ford ME, Havstad S, Vernon SW, Davis SD, Kroll D, Lamerato L, et al. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist 2006;46(4):545-50. - PubMed
-
- Ford ME, Randolph V, Hopkins-Johnson L, Eason SL, Havstad S, Jankowski M, et al. Design of a case management approach to enhance cancer screening trial retention among older African American men. Journal of Aging and Health 2004;16(5):39s-57s. - PubMed
Galaragga 2017 {published data only}
-
- Galárraga O, Sosa-Rubí SG, Kuo C, Gozalo P, González A, Saavedra B, et al. Punto Seguro: a randomized controlled pilot using conditional economic incentives to reduce sexually transmitted infection risks in Mexico. AIDS and Behavior 2017;21(12):3440-56. [DOI: 10.1007/s10461-017-1960-x] - DOI - PMC - PubMed
Gaurino 2006 {published data only}
Grabowski 1995 {published data only}
-
- Grabowski JP, Rhoades HP, Elk RP, Schmitz JP, Davis CP, Creson DM, et al. Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: two placebo-controlled, double-blind trials. Journal of Clinical Psychopharmacology 1995;15(3):163-74. - PubMed
Haines 2019 {published data only}
-
- Haines RH, Chalmers JR, Swinden R, Bradshaw LE, Montgomery AM, Williams HC. Maximising participant retention in a randomised prevention trial. Trials 2019;20(suppl.1):P-189.
Hall 1975 {published data only}
-
- Hall S, Hall R, Borden B, Hanson R. Follow-up strategies in the behavioural treatment of overweight. Behaviour Research and Therapy 1975;13:167-72. - PubMed
Hall 1978 {published data only}
-
- Hall S, Bass A, Monroe J. Continued contact and monitoring as follow-up strategies: a long-term study of obesity treatment. Addictive Behaviours 1978;3:139-47. - PubMed
Henderson 2019 {published data only}
-
- Henderson M, Waldram M, Thomas A, AlAlmmary F, Di Brito S, Ottman S, et al. Can financial incentives improve living donor follow-up?: a pilot randomized controlled trial. Transplant International 2019;32(suppl.2):199.
Hoang 2014 {published data only}
-
- Hoang U, Sheldenkar A, Leon J, Emmett E, Mckevitt C, Wolfe C. The effect of personalized care on long term loss to follow-up in a stroke register: WSC-0469. International Journal of Stroke 2014;9(Suppl 3):262.
Hoffman 1998 {published data only}
-
- Hoffman S, Burke A, Helzlsouer K, Comstock G. Controlled trial of the effect of length, incentives, and follow-up techniques on response to a mailed questionnaire. American Journal of Epidemiology 1998;148(10):1007-11. - PubMed
Hopkins 1983 {published data only}
-
- Hopkins K, Podolak J. Class-of-mail and the effects of monetary gratuity on the response rates of mailed questionnaires. Journal of Experimental Education 1983;51(4):169-70.
Hughes 1989 {published and unpublished data}
-
- Hughes JFR. Free reprints to increase the return of follow-up questionnaires. In: Abstracts from the Tenth Meeting of the Society of Clinical Trials, 1989 May 15-18; Minneapolis. Vol. 10. 1989:316-54.
Hunter 2018 {published data only}
-
- Hunter RF, Murray JM, Gough A, Tang J, Patterson C, French DP, et al. Effectiveness and cost-effectiveness of a loyalty scheme for physical activity behaviour change maintenance: results from a cluster randomised controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2018;15(1):127. [DOI: 10.1186/s12966-018-0758-1] - DOI - PMC - PubMed
Iglesias 2000 {published data only}
-
- Iglesias C, Torgerson D. Does length of questionnaire matter? A randomised trial of response rates to a mailed questionnaire. Journal of Health Services Research & Policy 2000;5(4):219-21. - PubMed
Iglesias 2001 {published data only}
-
- Iglesias CP, Birks YF, Torgerson DJ. Improving the measurement of quality of life in older people: the York SF-12. QJM: an International Journal of Medicine 2001;94:695-8. - PubMed
Johnson 2004 {published data only}
-
- Johnson G, Oxman M, Schmader K, Levin M, Concato J, Williams H, et al. Use of an automated telephone response system for follow-up in a long-term multi-centre clinical trial. Clinical Trials 2004;2:211.
Juraskova 2014 {published data only}
-
- Juraskova I, Butow P, Bonner C, Bell ML, Smith AN, Seccombe M, et al. Improving decision making about clinical trial participation - a randomised controlled trial of a decision aid for women considering participation in the IBIS-II breast cancer prevention trial. British Journal of Cancer 2014;111(1):1-7. [DOI: 10.1038/bjc.2014.144] - DOI - PMC - PubMed
Karras‐Jean Gilles 2019 {published data only}
-
- Karras-Jean Gilles J, Astuto J, Gjicali K, Allen LR. Sample retention in an urban context: exploring influential factors within a longitudinal randomized evaluation. American Journal of Evaluation 2019;40(2):268-90. [DOI: 10.1177/1098214017742719] - DOI
Katz 2001 {published data only}
-
- Katz K, El-Mohandes A, McNeely Johnson D, Jarrett M, Rose A, et al. Retention of low income mothers in a parenting intervention study. Journal of Community Health 2001;26(3):203-18. - PubMed
Kim 2020 {published data only}
-
- Kim M, Lee H, Kiang P, Aronowitz T, Sheldon LK, Shi L, et al. A Storytelling intervention in a mobile, web-based platform: a pilot randomized controlled trial to evaluate the preliminary effectiveness to promote human papillomavirus vaccination in Korean American college women. Health Education & Behavior 2020;47(2):258-63. [DOI: 10.1177/1090198119894589] - DOI - PMC - PubMed
Kiwanuka 2018 {published data only}
-
- Kiwanuka N, Mpendo J, Asiimwe S, Ssempiira J, Nalutaaya A, Nambuusi B, et al. A randomized trial to assess retention rates using mobile phone reminders versus physical contact tracing in a potential HIV vaccine efficacy population of fishing communities around Lake Victoria, Uganda. BMC Infectious Diseases 2018;18:591. [DOI: 10.1186/s12879-018-3475-0] - DOI - PMC - PubMed
Krammer 1986 {published data only}
Kuhlmann 2017 {published data only}
Lannin 2013 {published data only}
Leidy 2000 {published and unpublished data}
-
- Leidy NK, Legro M, Schmier J, Zyczynski T, McHorney C, Coyne K. Does order of administration affect subject response on generic and condition-specific measures of health related quality of life? Quality of Life Research 2000;9:337.
Leigh Brown 1997 {published and unpublished data}
Leighton 2018 {published data only}
Litchfield 2005 {published data only}
-
- Litchfield J, Freeman J, Schou H, Elsley M, Fuller R, Chubb B. Is the future for clinical trials internet-based? A cluster randomized clinical trial. Clinical Trials. 2005;1:72-9. - PubMed
Malden 2019 {published data only}
McAuley 1994 {published data only}
-
- McAuley E, Courneya K, Rudolph D, Cox CL. Enhancing exercise in middle aged males and females. Preventive Medicine 1994;23:498-506. - PubMed
McBee 2009 {published data only}
-
- McBee W, Clemons T, Chew E, SanGiovanni JP. A multi-level approach for retention of participants in a long-term clinical trial. Clinical Trials 2009;6:493-524.
Munoz 2017 {published data only}
Murray 2019 {published data only}
-
- Murray JM, French DP, Patterson CC, Kee F, Gough A, Tang J, et al. Predicting outcomes from engagement with specific components of an internet-based physical activity intervention with financial incentives: process analysis of a cluster randomized controlled trial. Journal of Medical Internet Research 2019;21(4):e11394. - PMC - PubMed
Murray 2020 {published data only}
Nicholas 2013 {published data only}
Nielson 2018 {published data only}
Novak 2019 {published data only}
-
- Novak LA, Belsher BE, Freed MC, McCutchan PK, Liu X, Evatt DP, et al. Impact of financial reimbursement on retention rates in military clinical trial research: a natural experiment within a multi-site randomized effectiveness trial with active duty service members. Contemporary Clinical Trials Communications 2019;15:100353. [DOI: 10.1016/j.conctc.2019.100353] - DOI - PMC - PubMed
Nuzzolese 2020 {published data only}
Parker 2019 {published data only}
-
- Parker A, Arundel C, Beard D, Bower P, Brocklehurst P, Coleman E, et al. PROMoting THE USE OF SWATs (PROMETHEUS): routinely embedding recruitment and retention interventions within randomised trials. Trials 2019;20(Suppl 1):46-7.
Paul 2011 {published data only}
-
- Paul J, Iveson T, Midgley R, Harkin A, Masterton M, Alexander L, et al. Choice of randomisation time-point in non-inferiority studies of reduced treatment duration: experience from the SCOT study. Trials 2011;12:1-2. [DOI: 10.1186/1745-6215-12-S1-A30] - DOI
Phiri 2019 {published data only}
-
- Phiri SC, Mudhune S, Prust ML, Haimbe P, Shakwelele H, Chisenga T, et al. Impact of the Umoyo mother-infant pair model on HIV-positive mothers? social support, perceived stigma and 12-month retention of their HIV-exposed infants in PMTCT care: evidence from a cluster randomized controlled trial in Zambia. Trials 2019;20(1):505. [DOI: 10.1186/s13063-019-3617-8] - DOI - PMC - PubMed
Pieper 2018 {published data only}
Poling 2006 {published data only}
-
- Poling J, Oliveto A, Petry N, Sofuoglu M, Gonsai K, Gonzalez G, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Archives of General Psychiatry 2006;63:219-28. - PubMed
Price 2019 {published data only}
Puffer 2004 {published data only}
-
- Puffer S, Porthouse J, Birks Y, Morton V, Torgerson D. Increasing response rates to postal questionnaires: a randomised trial of variations in design. Journal of Health Services Research and Policy 2004;9(4):213?7. - PubMed
Rhoades 1998 {published data only}
Roberts 2000 {published data only}
Rodgers 2019a {published data only}
-
- Rodgers S, Sbizzera I, Cockayne S, Fairhurst C, Lamb SE, Vernon W, et al. A study update newsletter or Post-it® note did not increase postal questionnaire response rates in a falls prevention trial: an embedded randomised factorial trial. F1000Research 2019;7:1083. [DOI: 10.12688/f1000research.14591.2] - DOI - PMC - PubMed
Rodgers 2019b {published data only}
-
- Rodgers M, Meisel Z, Wiebe D, Crits-Christoph P, Rhodes KV. Wireless participant incentives using reloadable bank cards to increase clinical trial retention with abused women drinkers: a natural experiment. Journal of Interpersonal Violence 2019;34(13):2774-96. [DOI: 10.1177/0886260516662849] - DOI - PMC - PubMed
Rolfson 2011 {published data only}
-
- Rolfson O, Salomonsson R, Dahlberg LE, Garellick G. Internet-based follow-up questionnaire for measuring patient-reported outcome after total hip replacement surgery?reliability and response rate. Value in Health 2011;14(2):316-21. [DOI: 10.1016/j.jval.2010.08.004 ] - PubMed
Sano 2013 {published data only}
Schmitz 2005 {published data only}
-
- Schmitz J, Sayre S, Stotts A, Rothfleisch J, Mooney M. Medication compliance during a smoking cessation clinical trial: a brief intervention using MEMS feedback. Journal of Behavioral Medicine 2005;28(2):139-47. - PubMed
Shulman 2019 {published data only}
Smeeth 2001ab {published data only}
-
- Smeeth L, Fletcher AE, Stirling S, Nunes M, Breeze E, Ng E, et al. Randomised comparison of three methods of administering a screening questionnaire to elderly people: findings from the MRC trial of the assessment and management of older people in the community. BMJ 2001;323(7326):1403. - PMC - PubMed
Smith 2015 {published data only}
Stoner 1998 {published data only}
Svoboda 2001 {published and unpublished data}
-
- Svoboda P. A comparison of two questionnaires for assessing outcome after head injury. Personal email communication from P Edwards in April 2010.
Tariq 2019 {published data only}
Tassopoulos 2007 {published data only}
-
- Tassopoulos C. The challenges of follow-up in a multi-centre randomised controlled trial. Clinical Trials 2007;4:371-455.
Trevena 2006 {published data only}
von Allmen 2019 {published data only}
Wagstaff 2019 {published data only}
Wensing 2005 {published data only}
Weston 2017 {published data only}
Wood 2015 {published data only}
-
- Wood J, Bruhn H, Cook JA, McDonald A, Norrie J, Watson AJ. PTU-225 Strategies to improve response rates to patient reported outcome measures in a surgical rct. Gut 2015;64:A161-2. [DOI: 10.1136/gutjnl-2015-309861.340] - DOI
Wood 2017 {published data only}
-
- Wood J, Cook JA, Hudson J, McDonald A, Bruhn H, Watson AJ. Do higher monetary incentives improve response rates part-way through a randomised control trial? Trials 2017;18(Suppl 1):P65. [DOI: 10.1186/1745-6215-16-S2-P123 ]
Wu 1997 {published and unpublished data}
-
- Wu AW, Jacobson DL, Berzon RA, Revicki DA, Horst C, Fichtenbaum CJ, et al. The effect of mode of administration on medical outcomes study health ratings and EuroQol scores in AIDS. Quality of Life Research 1997;6(1):3-10. - PubMed
References to studies awaiting assessment
Letley 2000 {published data only}
-
- Letley L, Garratt A. Questionnaire length and internal reliability: a randomised study. In: Society for Social Medicine 43rd Annual Scientific Meeting; 2000 6-8 Sept; Norwich. 2000.
Sutherland 1996 {published data only}
References to ongoing studies
SWAT #100 {unpublished data only}
-
- O'Neill L, Knapp P, Doyle S, Guinan E, Hussey J. SWAT 100: Patient and family co-developed participant information to improve recruitment rates, retention, and patient understanding of a randomised trial. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog.... - PMC - PubMed
SWAT #105 {unpublished data only}
-
- Hynes S, Dwyer C, Joyce R. SWAT 105: Effects of a patient-designed-and-informed participant information sheet versus a standard, researcher-designed information sheet on recruitment to a randomised trial. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #107 {unpublished data only}
-
- Shiely F. SWAT 107: Effects of a multi-trial programmable animation platform on the efficiency and success of pre-screening and subsequent recruitment to a randomised trial. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #109 {unpublished data only}
-
- Parker A, Brealey S, Sharma HK, Welch C, Keding A, Flett L, Hunkins D, Witts J, McDaid C. SWAT 109: The effectiveness of a text message reminder which participants can respond to, compared with a?no reply? text message on questionnaire response rates. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT#110 {unpublished data only}
-
- Ooms A. SWAT 110: Printing the primary outcomE on Pink PapER versus standard paper to increase participant engagement to postal questionnaires (PEPPER). https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT#112 {unpublished data only}
-
- Woodford J & von Essen L. SWAT 112: Effects on recruitment of a personalised compared with a standard study invitation letter. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #119 {unpublished data only}
-
- Arundel C, Chetter I, Fairhurst C, Joshi K, McCaffery J, Mott A, Wilkinson J. SWAT 119: Effects on retention of giving trial participants a thank you card following each study visit.. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #121 {unpublished data only}
-
- Dhanjal G. SWAT 121: What are the effects on retention and follow-up of courtesy telephone calls versus postcards to trial participants following enrolment? https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #51 {unpublished data only}
-
- Agus A. SWAT 51: Promoting group identity to improve questionnaire return rate. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #54 {unpublished data only}
-
- Anand R. SWAT 54: Giving trial participants a thank you note or card after each study visit. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #63 {unpublished data only}
-
- Azuara-Blanco A & Clarke M. SWAT 63: Does local radio and social media advertisement increase recruitment? https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #79 {unpublished data only}
-
- Backhouse M, Torgerson D, Parker A, Cockayne S. SWAT 79: Effect of birthday cards with or without nudge on retention and data completion rates in trials involving children. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #81 {unpublished data only}
-
- BenSaaud A, Tawfick W, Jordan F. SWAT 81: A Telephone Reminder to Enhance Adherence to Interventions in Randomised Trials. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog.... - PubMed
SWAT #82 {published data only}
-
- Treweek S, Gillies K, Innes K, MacLennan G. SWAT 82: Sending Christmas cards to trial participants to improve retention.. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog.... - PMC - PubMed
SWAT #86 {unpublished data only}
-
- Sutton C, Cotterill S, Forshaw, D Rhodes S, Hammond A. SWAT 86: Advance notification of trial participants before outcome data collection to improve retention. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #87 {unpublished data only}
-
- Hopewell S, Cureton L, Greenall G. SWAT 87: Do participants complete the original or the reminder postal follow up questionnaire? https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #89 {unpublished data only}
-
- Starr K, McRae D, Gillies K, Cooper C, Wolfe A. SWAT 89: Including a theoretically informed leaflet in a participant takehome pack of questionnaires to increase response rate. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #92 {unpublished data only}
-
- Tew G, Tilbrook H, Paul S, Howe L, Parker A, Bell K. SWAT 92: Pen incentive to enhance retention in a randomised trial. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
SWAT #97 {unpublished data only}
-
- kKnapp P. SWAT 97: TRECA (TRials Engagement in Children and Adolescents). https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodolog....
Additional references
Bensaaud 2020
-
- Bensaaud A, Gibson I, Jones J, Flaherty G, Sultan S, Tawfick W, Jordan F. A telephone reminder to enhance adherence to interventions in cardiovascular randomized trials: a protocol for a study within a trial (SWAT). Journal of Evidence-based Medicine 2020;13(1):81-4. [DOI: 10.1111/jebm.12375] - DOI - PubMed
Booker 2011
Brunsdon 2019
-
- Brunsdon D, Biesty L, Brocklehurst P, Brueton V, Devane D, Elliott J, et al. What are the most important unanswered research questions in trial retention? A James Lind Alliance Priority Setting Partnership: the PRioRiTy II (Prioritising Retention in Randomised Trials) study. Trials 2019;20(1):593. [DOI: 10.1186/s13063-019-3687-7] - DOI - PMC - PubMed
Chan 2013
Edwards 2009
Egger 1997
Fewtrell 2008
-
- Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Archives of Disease in Childhood 2008;93(6):458-61. - PubMed
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck?Ytter Y, Alonso?Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [DOI: 10.1136/bmj.39489.470347.AD] - DOI - PMC - PubMed
Higgins 2003
Higgins 2008a
-
- Higgins JP, Altman D. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editors(s). Cochrane Handbook of Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:188-241.
Higgins 2008b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:482-529.
Hoffman 2014
-
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI: ] - PubMed
Hollis 1999
Madurasinghe 2016
Moher 2010
Murray 2009
PROMETHEUS
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Robotham 2016
Santesso 2020
Schulz 2002
-
- Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet 2002;359(9308):781-5. - PubMed
Skea 2019
Sterne 2008
-
- Sterne J, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:297-333.
SWAT
-
- SWAT repository. www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearc... Accessed on 1 December 2020.
Treweek 2018
Treweek 2020b
Trial Forge
-
- Trial Forge - A systematic way to improve trial efficiency. wwww.trialforge.org.
Walsh 2014
Walsh 2015
Walters 2016
-
- Walters SJ, Bonacho Dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 2017;7:3. [DOI: 10.1136/bmjopen-2016-015276] - DOI - PMC - PubMed
References to other published versions of this review
Brueton 2011
-
- Brueton VC, Rait G, Tierney J, Meredith S, Darbyshire J, Harding S, et al. Strategies to reduce attrition in randomised trials. Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No: MR000032. [DOI: 10.1002/14651858.MR000032] - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

