A smartphone based method for mouse fundus imaging
- PMID: 33675778
- PMCID: PMC8087636
- DOI: 10.1016/j.exer.2021.108530
A smartphone based method for mouse fundus imaging
Abstract
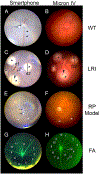
Noninvasive in vivo imaging of the mouse retina is essential for eye research. However, imaging the mouse fundus is challenging due to its small size and requires specialized equipment, maintenance, and training. These issues hinder the routine evaluation of the mouse retina. In this study, we developed a noncontact imaging system consisting of a smartphone, a 90D condensing lens, a homemade light diaphragm, a tripod, and a Bluetooth remote. With minimal training, examiners were able to capture fundus images from the mouse retina. We also found that fundus images captured using our system from wild type mice, mice with laser-induced retinal injury, and a mouse model of retinitis pigmentosa showed a quality similar to those captured using a commercial fundus camera. These images enabled us to identify normal structures and pathological changes in the mouse retina. Additionally, fluorescein angiography was possible with the smartphone system. We believe that the smartphone imaging system is low cost, simple, accessible, easy to operate, and suitable for the routine screening and examination of the mouse eye.
Keywords: Fluorescein angiography; Fundus imaging; Laser-induced retinal injury; Mouse retina; Retinitis pigmentosa; Smartphone.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures

References
-
- Calderone L, Grimes P, Shalev M, 1986. Acute reversible cataract induced by xylazine and by ketamine-xylazine anesthesia in rats and mice. Exp Eye Res 42, 331–337. - PubMed
-
- Cohan BE, Pearch AC, Jokelainen PT, Bohr DF, 2003. Optic disc imaging in conscious rats and mice. Invest Ophthalmol Vis Sci 44, 160–163. - PubMed
-
- DiLoreto D Jr., Grover DA, del Cerro C, del Cerro M, 1994. A new procedure for fundus photography and fluorescein angiography in small laboratory animal eyes. Curr Eye Res 13, 157–161. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

