Hypoxia promotes erythroid differentiation through the development of progenitors and proerythroblasts
- PMID: 33675821
- PMCID: PMC8102433
- DOI: 10.1016/j.exphem.2021.02.012
Hypoxia promotes erythroid differentiation through the development of progenitors and proerythroblasts
Abstract
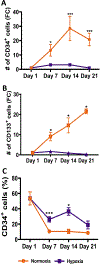
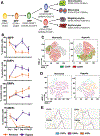
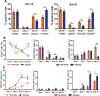
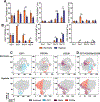
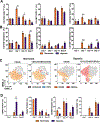
Oxygen is a critical noncellular component of the bone marrow microenvironment that plays an important role in the development of hematopoietic cell lineages. In this study, we investigated the impact of low oxygen (hypoxia) on ex vivo myeloerythroid differentiation of human cord blood-derived CD34+ hematopoietic stem and progenitor cells. We characterized the culture conditions to demonstrate that low oxygen inhibits cell proliferation and causes a metabolic shift in the stem and progenitor populations. We found that hypoxia promotes erythroid differentiation by supporting the development of progenitor populations. Hypoxia also increases the megakaryoerythroid potential of the common myeloid progenitors and the erythroid potential of megakaryoerythroid progenitors and significantly accelerates maturation of erythroid cells. Specifically, we determined that hypoxia promotes the loss of CD71 and the appearance of the erythroid markers CD235a and CD239. Further, evaluation of erythroid populations revealed a hypoxia-induced increase in proerythroblasts and in enucleation of CD235a+ cells. These results reveal the extensive role of hypoxia at multiple steps during erythroid development. Overall, our work establishes a valuable model for further investigations into the relationship between erythroid progenitors and/or erythroblast populations and their hypoxic microenvironment.
Copyright © 2021 ISEH -- Society for Hematology and Stem Cells. Published by Elsevier Inc. All rights reserved.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

