Human limbal epithelial stem cell regulation, bioengineering and function
- PMID: 33676006
- PMCID: PMC8428188
- DOI: 10.1016/j.preteyeres.2021.100956
Human limbal epithelial stem cell regulation, bioengineering and function
Abstract
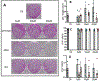
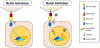
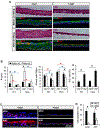
The corneal epithelium is continuously renewed by limbal stem/progenitor cells (LSCs), a cell population harbored in a highly regulated niche located at the limbus. Dysfunction and/or loss of LSCs and their niche cause limbal stem cell deficiency (LSCD), a disease that is marked by invasion of conjunctival epithelium into the cornea and results in failure of epithelial wound healing. Corneal opacity, pain, loss of vision, and blindness are the consequences of LSCD. Successful treatment of LSCD depends on accurate diagnosis and staging of the disease and requires restoration of functional LSCs and their niche. This review highlights the major advances in the identification of potential LSC biomarkers and components of the LSC niche, understanding of LSC regulation, methods and regulatory standards in bioengineering of LSCs, and diagnosis and staging of LSCD. Overall, this review presents key points for researchers and clinicians alike to consider in deepening the understanding of LSC biology and improving LSCD therapies.
Keywords: Anterior segment coherence tomography; Cell therapy; In vivo laser scanning confocal microscopy; Limbal stem cell; Limbal stem cell deficiency; Notch signaling pathway; Small molecules; Wnt signaling pathway.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures



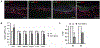









References
-
- Alok A, Lei Z, Jagannathan NS, Kaur S, Harmston N, Rozen SG, Tucker-Kellogg L, Virshup DM, 2017. Wnt proteins synergize to activate beta-catenin signaling. J Cell Sci 130, 1532–1544. - PubMed
-
- Andersson ER, Sandberg R, Lendahl U, 2011. Notch signaling: simplicity in design, versatility in function. Development 138, 3593–3612. - PubMed
-
- Ang LP, Sotozono C, Koizumi N, Suzuki T, Inatomi T, Kinoshita S, 2007. A comparison between cultivated and conventional limbal stem cell transplantation for Stevens-Johnson syndrome. Am J Ophthalmol 143, 178–180. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

