Computerized spermatogenesis staging (CSS) of mouse testis sections via quantitative histomorphological analysis
- PMID: 33676102
- PMCID: PMC8046964
- DOI: 10.1016/j.media.2020.101835
Computerized spermatogenesis staging (CSS) of mouse testis sections via quantitative histomorphological analysis
Abstract
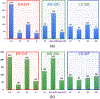
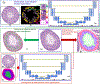
Spermatogenesis in mammals is a cyclic process of spermatogenic cell development in the seminiferous epithelium that can be subdivided into 12 subsequent stages. Histological staging analysis of testis sections, specifically of seminiferous tubule cross-sections, is the only effective method to evaluate the quality of the spermatogenic process and to determine developmental defects leading to infertility. Such staging analysis, however, is tedious and time-consuming, and it may take a long time to become proficient. We now have developed a Computerized Staging system of Spermatogenesis (CSS) for mouse testis sections through learning of an expert with decades of experience in mouse testis staging. The development of the CSS system comprised three major parts: 1) Developing computational image analysis models for mouse testis sections; 2) Automated classification of each seminiferous tubule cross-section into three stage groups: Early Stages (ES: stages I-V), Middle Stages (MS: stages VI-VIII), and Late Stages (LS: stages IV-XII); 3) Automated classification of MS into distinct stages VI, VII-mVIII, and late VIII based on newly developed histomorphological features. A cohort of 40 H&E stained normal mouse testis sections was built according to three modules where 28 cross-sections were leveraged for developing tubule region segmentation, spermatogenic cells types and multi-concentric-layers segmentation models. The rest of 12 testis cross-sections, approximately 2314 tubules whose stages were manually annotated by two expert testis histologists, served as the basis for developing the CSS system. The CSS system's accuracy of mean and standard deviation (MSD) in identifying ES, MS, and LS were 0.93 ± 0.03, 0.94 ± 0.11, and 0.89 ± 0.05 and 0.85 ± 0.12, 0.88 ± 0.07, and 0.96 ± 0.04 for one with 5 years of experience, respectively. The CSS system's accuracy of MSD in identifying stages VI, VII-mVIII, and late VIII are 0.74 ± 0.03, 0.85 ± 0.04, and 0.78 ± 0.06 and 0.34 ± 0.18, 0.78 ± 0.16, and 0.44 ± 0.25 for one with 5 years of experience, respectively. In terms of time it takes to collect these data, it takes on average 3 hours for a histologist and 1.87 hours for the CSS system to finish evaluating an entire testis section (computed with a PC (I7-6800k 4.0 GHzwith 32GB of RAM & 256G SSD) and a Titan 1080Ti GPU). Therefore, the CSS system is more accurate and faster compared to a human histologist in staging, and further optimization and development will not only lead to a complete staging of all 12 stages of mouse spermatogenesis but also could aid in the future diagnosis of human infertility. Moreover, the top-ranking histomorphological features identified by the CSS classifier are consistent with the primary features used by histologists in discriminating stages VI, VII-mVIII, and late VIII.
Keywords: Computerized staging of spermatogenesis; Deep learning; Mouse testicular section images; Mouse testis histology; Seminiferous tubules; Sperm development; Spermatogenic cell segmentation.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





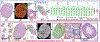



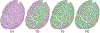

Similar articles
-
Intraoperative frozen section analysis for the diagnosis of early stage ovarian cancer in suspicious pelvic masses.Cochrane Database Syst Rev. 2016 Mar 1;3(3):CD010360. doi: 10.1002/14651858.CD010360.pub2. Cochrane Database Syst Rev. 2016. PMID: 26930463 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
A comparison of spermatogenesis between flies and men-conserved processes of male gamete production.Hum Reprod Update. 2025 Aug 13:dmaf018. doi: 10.1093/humupd/dmaf018. Online ahead of print. Hum Reprod Update. 2025. PMID: 40802929
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
Cited by
-
Development of a procedure for isolation, identification and quality assessment of bovine spermatids and evaluation of their fertilizing ability in vitro.Front Bioeng Biotechnol. 2025 May 16;13:1581019. doi: 10.3389/fbioe.2025.1581019. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40453182 Free PMC article.
-
Single-Cell Transcriptome Sequencing Reveals Molecular Expression Differences and Marker Genes in Testes during the Sexual Maturation of Mongolian Horses.Animals (Basel). 2024 Apr 23;14(9):1258. doi: 10.3390/ani14091258. Animals (Basel). 2024. PMID: 38731262 Free PMC article.
-
Deep-learning-based automated prediction of mouse seminiferous tubule stage by using bright-field microscopy.Sci Rep. 2025 Jul 1;15(1):21849. doi: 10.1038/s41598-025-06727-x. Sci Rep. 2025. PMID: 40594622 Free PMC article.
-
Proliferation and Apoptosis of Cat (Felis catus) Male Germ Cells during Breeding and Non-Breeding Seasons.Vet Sci. 2022 Aug 20;9(8):447. doi: 10.3390/vetsci9080447. Vet Sci. 2022. PMID: 36006362 Free PMC article.
-
Wasserstein HOG: Local Directionality Extraction via Optimal Transport.IEEE Trans Med Imaging. 2024 Mar;43(3):916-927. doi: 10.1109/TMI.2023.3325295. Epub 2024 Mar 5. IEEE Trans Med Imaging. 2024. PMID: 37874704 Free PMC article.
References
-
- Ahmed EA, de Rooij DG, 2009. Staging of Mouse Seminiferous Tubule Cross-Sections. Methods in Molecular Biology, 558. Humana Press, Totowa, NJ, pp. 263–277. - PubMed
-
- Clermont Y, 1972. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev 52 (1), 198–236. - PubMed
-
- Crosier M, Griffin LD, 2010. Using basic image features for texture classification. Int J Comput Vis 88 (3), 447–460.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

