Can changes in skin impedance be used to monitor sedation after midazolam and during recovery from anesthesia?
- PMID: 33676384
- PMCID: PMC8820580
- DOI: 10.33549/physiolres.934621
Can changes in skin impedance be used to monitor sedation after midazolam and during recovery from anesthesia?
Abstract
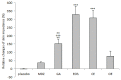
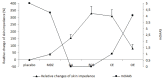
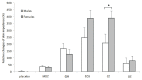
It has been suggested that sympathetic activity, measured as changes in electrical skin impedance (SI), can be used to assess the adequacy of general anesthesia. Our prospective study investigated if measurements of skin impedance can determine levels of sedation induced by midazolam. Twenty-seven patients scheduled for arthroscopy requiring general anesthesia were served as their own control. These were blinded to the order of injections by telling them that they will be randomly administered a placebo (saline) orsedative agent. A DM 3900 multimeter was used for SI measurements. The degree of sedation was measured using the modified Observer's Assessment of Alertness and Sedation (mOAAS) scale. Resting SI values were noted, and all participants were then administered the placebo followed 5 min later by midazolam 2 mg i.v. Five min after that, patients were administered standard general anesthesia with propofol, oxygen, nitrous oxide 60 %, and isoflurane 1 MAC via a laryngeal mask, and sufentanil 5 - 10 µg. SI significantly increased after administration of midazolam and induction of anesthesia. There were no significant differences between pre-administration (baseline) and placebo and end of surgery and end of anesthesia with closed eyes. There were highly significant differences (p<0.001) between pre-administration vs. midazolam, placebo vs. midazolam, pre-administration vs. induction of anesthesia. We found slight correlation between mOAAS and SI. There were no significant changes between the end of surgery and the end of anesthesia with closed eyes, but SI significantly decreased (p<0.01) after eyes opened.
Conflict of interest statement
There is no conflict of interest.
Figures
Similar articles
-
Midazolam premedication increases sedation but does not prolong discharge times after brief outpatient general anesthesia for laparoscopic tubal sterilization.Anesth Analg. 1997 Aug;85(2):301-5. doi: 10.1097/00000539-199708000-00011. Anesth Analg. 1997. PMID: 9249104 Clinical Trial.
-
Comparison of the sedation and recovery profiles of Ro 48-6791, a new benzodiazepine, and midazolam in combination with meperidine for outpatient endoscopic procedures.Anesth Analg. 1999 Oct;89(4):893-8. doi: 10.1097/00000539-199910000-00014. Anesth Analg. 1999. PMID: 10512261 Clinical Trial.
-
Monitored anesthesia care with dexmedetomidine: a prospective, randomized, double-blind, multicenter trial.Anesth Analg. 2010 Jan 1;110(1):47-56. doi: 10.1213/ane.0b013e3181ae0856. Epub 2009 Aug 27. Anesth Analg. 2010. PMID: 19713256 Clinical Trial.
-
Breathing patterns and levels of consciousness in children during administration of nitrous oxide after oral midazolam premedication.J Oral Maxillofac Surg. 1997 Dec;55(12):1372-7; discussion 1378-9. doi: 10.1016/s0278-2391(97)90630-3. J Oral Maxillofac Surg. 1997. PMID: 9393395
-
Effect of Midazolam in Addition to Propofol and Opiate Sedation on the Quality of Recovery After Colonoscopy: A Randomized Clinical Trial.Anesth Analg. 2020 Sep;131(3):741-750. doi: 10.1213/ANE.0000000000004620. Anesth Analg. 2020. PMID: 31922999 Clinical Trial.
Cited by
-
Sleuthing subjectivity: a review of covert measures of consciousness.Nat Rev Neurosci. 2025 Aug;26(8):476-496. doi: 10.1038/s41583-025-00934-1. Epub 2025 May 23. Nat Rev Neurosci. 2025. PMID: 40410390 Review.
References
-
- CHERNIK DA, GILLINGS D, LAINE H, HENDLER J, SILVER JM, DAVIDSON AB, SCHWAM EM, SIEGEL JL. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–251. doi: 10.1097/00004714-199008000-00003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
