Concept and development of an interactive tool for trial recruitment planning and management
- PMID: 33676535
- PMCID: PMC7936448
- DOI: 10.1186/s13063-021-05112-z
Concept and development of an interactive tool for trial recruitment planning and management
Abstract
Background: Predicting and monitoring recruitment in large, complex trials is essential to ensure appropriate resource management and budgeting. In a novel partnership between clinical trial investigators of the South African Medical Research Council and industrial engineers from the Stellenbosch University Health Systems Engineering and Innovation Hub, we developed a trial recruitment tool (TRT). The objective of the tool is to serve as a computerised decisions-support system to aid the planning and management phases of the trial recruitment process.
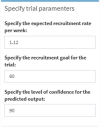
Method: The specific requirements of the TRT were determined in several workshops between the partners. A Poisson process simulation model was formulated and incorporated in the TRT to predict the recruitment duration. The assumptions underlying the model were made in consultation with the trial team at the start of the project and were deemed reasonable. Real-world data extracted from a current cluster trial, Project MIND, based in 24 sites in South Africa was used to verify the simulation model and to develop the monitoring component of the TRT.
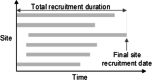
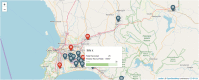
Results: The TRT comprises a planning and monitoring component. The planning component generates different trial scenarios for predicted trial recruitment duration based on user inputs, e.g. number of sites, initiation delays. The monitoring component uses and analyses the data retrieved from the trial management information system to generate different levels of information, displayed visually on an interactive, user-friendly dashboard. Users can analyse the results at trial or site level, changing input parameters to see the resultant effect on the duration of trial recruitment.
Conclusion: This TRT is an easy-to-use tool that assists in the management of the trial recruitment process. The TRT has potential to expedite improved management of clinical trials by providing the appropriate information needed for the planning and monitoring of the trial recruitment phase. This TRT extends prior tools describing historic recruitment only to using historic data to predict future recruitment. The broader project demonstrates the value of collaboration between clinicians and engineers to optimise their respective skillsets.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources