Human neural stem cell-derived extracellular vesicles mitigate hallmarks of Alzheimer's disease
- PMID: 33676561
- PMCID: PMC7937214
- DOI: 10.1186/s13195-021-00791-x
Human neural stem cell-derived extracellular vesicles mitigate hallmarks of Alzheimer's disease
Abstract
Background: Regenerative therapies to mitigate Alzheimer's disease (AD) neuropathology have shown very limited success. In the recent era, extracellular vesicles (EVs) derived from multipotent and pluripotent stem cells have shown considerable promise for the treatment of dementia and many neurodegenerative conditions.
Methods: Using the 5xFAD accelerated transgenic mouse model of AD, we now show the regenerative potential of human neural stem cell (hNSC)-derived EVs on the neurocognitive and neuropathologic hallmarks in the AD brain. Two- or 6-month-old 5xFAD mice received single or two intra-venous (retro-orbital vein, RO) injections of hNSC-derived EVs, respectively.
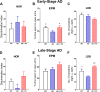
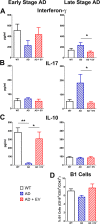
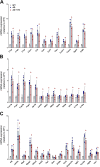
Results: RO treatment using hNSC-derived EVs restored fear extinction memory consolidation and reduced anxiety-related behaviors 4-6 weeks post-injection. EV treatment also significantly reduced dense core amyloid-beta plaque accumulation and microglial activation in both age groups. These results correlated with partial restoration of homeostatic levels of circulating pro-inflammatory cytokines in the AD mice. Importantly, EV treatment protected against synaptic loss in the AD brain that paralleled improved cognition. MiRNA analysis of the EV cargo revealed promising candidates targeting neuroinflammation and synaptic function.
Conclusions: Collectively, these data demonstrate the neuroprotective effects of systemic administration of stem cell-derived EVs for remediation of behavioral and molecular AD neuropathologies.
Keywords: Alzheimer’s disease; Extracellular vesicle; Inflammatory response; Neural stem cell.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

