Polyphosphates induce amyloid fibril formation of α-synuclein in concentration-dependent distinct manners
- PMID: 33676889
- PMCID: PMC8059054
- DOI: 10.1016/j.jbc.2021.100510
Polyphosphates induce amyloid fibril formation of α-synuclein in concentration-dependent distinct manners
Abstract
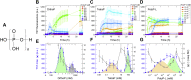
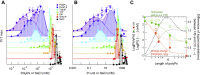
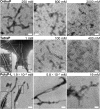
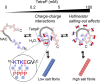
Polyphosphates (polyPs), chains of phosphate residues found in species across nature from bacteria to mammals, were recently reported to accelerate the amyloid fibril formation of many proteins. How polyPs facilitate this process, however, remains unknown. To gain insight into their mechanisms, we used various physicochemical approaches to examine the effects of polyPs of varying chain lengths on ultrasonication-dependent α-synuclein (α-syn) amyloid formation. Although orthophosphate and diphosphate exhibited a single optimal concentration of amyloid formation, triphosphate and longer-chain phosphates exhibited two optima, with the second at a concentration lower than that of orthophosphate or diphosphate. The second optimum decreased markedly as the polyP length increased. This suggested that although the optima at lower polyP concentrations were caused by interactions between negatively charged phosphate groups and the positive charges of α-syn, the optima at higher polyP concentrations were caused by the Hofmeister salting-out effects of phosphate groups, where the effects do not depend on the net charge. NMR titration experiments of α-syn with tetraphosphate combined with principal component analysis revealed that, at low tetraphosphate concentrations, negatively charged tetraphosphates interacted with positively charged "KTK" segments in four KTKEGV repeats located at the N-terminal region. At high concentrations, hydrated tetraphosphates affected the surface-exposed hydrophilic groups of compact α-syn. Taken together, our results suggest that long-chain polyPs consisting of 60 to 70 phosphates induce amyloid formation at sub-μM concentrations, which are comparable with the concentrations of polyPs in the blood or tissues. Thus, these findings may identify a role for polyPs in the pathogenesis of amyloid-related diseases.
Keywords: amyloid; biophysics; nuclear magnetic resonance (NMR); polyphosphate; protein aggregation; solubility; supersaturation; synuclein.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Riek R., Eisenberg D.S. The activities of amyloids from a structural perspective. Nature. 2016;539:227–235. - PubMed
-
- Sipe J.D., Benson M.D., Buxbaum J.N., Ikeda S.I., Merlini G., Saraiva M.J., Westermark P. Amyloid fibril proteins and amyloidosis: Chemical identification and clinical classification International Society of Amyloidosis 2016 nomenclature guidelines. Amyloid. 2016;23:209–213. - PubMed
-
- Chiti F., Dobson C.M. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu. Rev. Biochem. 2017;86:27–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

