An altered gut microbiota in duck-origin parvovirus infection on cherry valley ducklings is associated with mucosal barrier dysfunction
- PMID: 33677399
- PMCID: PMC7940990
- DOI: 10.1016/j.psj.2021.101021
An altered gut microbiota in duck-origin parvovirus infection on cherry valley ducklings is associated with mucosal barrier dysfunction
Abstract
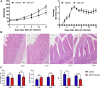
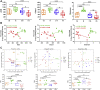
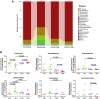
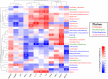
Duck-origin parvovirus disease is an epidemic disease mainly caused by duck-origin goose parvovirus (D-GPV), which is characterized by beak atrophy and dwarfism syndrome. Its main symptoms are persistent diarrhea, skeletal dysplasia, and growth retardation. However, the pathogenesis of Cherry Valley ducks infected by D-GPV has not been studied thoroughly. To perceive the distribution of D-GPV in the intestinal tract, intestinal morphological development, intestinal permeability, inflammatory cytokines in Cherry Valley ducks, and expression of tight junction protein, the D-GPV infection was given intramuscularly. Illumina MiSeq sequencing technology was used to analyze the diversity and structure of ileum flora and content of short-chain fatty acids of its metabolites. To investigate the relationship between intestinal flora changes and intestinal barrier function after D-GPV infection on Cherry Valley ducks is of great theoretical and practical significance for further understanding the pathogenesis of D-GPV and the structure of intestinal flora in ducks. The results showed that D-GPV infection was accompanied by intestinal inflammation and barrier dysfunction. At this time, the decrease of a large number of beneficial bacteria and the content of short-chain fatty acids in intestinal flora led to the weakening of colonization resistance of the intestinal flora and the accumulation of potentially pathogenic bacteria, which would aggravate the negative effect of D-GPV damage to the intestinal tract. Furthermore, a significant increase in Unclassified_S24-7 and decrease in Streptococcus was observed in D-GPV persistent, indicating the disruption in the structure of gut microbiota. Notably, the shift of microbiota was associated with the transcription of tight-junction protein and immune-associated cytokines. These results indicate that altered ileum microbiota, intestinal barrier, and immune dysfunction are associated with D-GPV infection. Therefore, there is a relationship between the intestinal barrier dysfunction and dysbiosis caused by D-GPV, but the specific mechanism needs to be further explored.
Keywords: D-GPV; gut microbiome; immune dysfunction; intestinal barrier dysfunction.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Adegunloye D.V. Microorganisms associated with poultry faeces. J. Food Agric. Environ. 2006;4:41–42.
-
- Aijaz S., Balda M.S., Matter K. Tight junctions: molecular architecture and function. Int. Rev. Cytol. 2006;248:261–298. - PubMed
-
- Applegate T.J., Karcher D.M., Lilburn M.S. Comparative development of the small intestine in the Turkey poult and Pekin duckling. Poult. Sci. 2005;84:426. - PubMed
-
- Applegate T.J., Ladwig E., Weissert L., Lilburn M.S. Effect of hen age on intestinal development and glucose tolerance of the Pekin duckling. Poult. Sci. 1999;78:1485–1492. - PubMed
-
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008;8:411–420. - PubMed
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

