Impact of Anti-PD-1 and Anti-CTLA-4 on the Human Immunodeficiency Virus (HIV) Reservoir in People Living With HIV With Cancer on Antiretroviral Therapy: The AIDS Malignancy Consortium 095 Study
- PMID: 33677480
- PMCID: PMC8492152
- DOI: 10.1093/cid/ciaa1530
Impact of Anti-PD-1 and Anti-CTLA-4 on the Human Immunodeficiency Virus (HIV) Reservoir in People Living With HIV With Cancer on Antiretroviral Therapy: The AIDS Malignancy Consortium 095 Study
Abstract
Background: Antibodies to programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) may perturb human immunodeficiency virus (HIV) persistence during antiretroviral therapy (ART) by reversing HIV latency and/or boosting HIV-specific immunity, leading to clearance of infected cells. We tested this hypothesis in a clinical trial of anti-PD-1 alone or in combination with anti-CTLA-4 in people living with HIV (PLWH) and cancer.
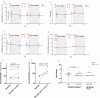
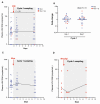
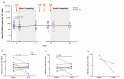
Methods: This was a substudy of the AIDS Malignancy Consortium 095 Study. ART-suppressed PLWH with advanced malignancies were assigned to nivolumab (anti-PD-1) with or without ipilimumab (anti-CTLA-4). In samples obtained preinfusion and 1 and 7 days after the first and fourth doses of immune checkpoint blockade (ICB), we quantified cell-associated unspliced (CA-US) HIV RNA and HIV DNA. Plasma HIV RNA was quantified during the first treatment cycle. Quantitative viral outgrowth assay (QVOA) to estimate the frequency of replication-competent HIV was performed before and after ICB for participants with samples available.
Results: Of 40 participants, 33 received nivolumab and 7 nivolumab plus ipilimumab. Whereas CA-US HIV RNA did not change with nivolumab monotherapy, we detected a median 1.44-fold increase (interquartile range, 1.16-1.89) after the first dose of nivolumab and ipilimumab combination therapy (P = .031). There was no decrease in the frequency of cells containing replication-competent HIV, but in the 2 individuals on combination ICB for whom we had longitudinal QVOA, we detected decreases of 97% and 64% compared to baseline.
Conclusions: Anti-PD-1 alone showed no effect on HIV latency or the latent HIV reservoir, but the combination of anti-PD-1 and anti-CTL-4 induced a modest increase in CA-US HIV RNA and may potentially eliminate cells containing replication-competent HIV.
Clinical trials registration: NCT02408861.
Keywords: HIV; HIV latency; anti–CTLA-4; anti–PD-1.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 2019; 37:457–95. - PubMed
-
- Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 2006; 12:1198–202. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

